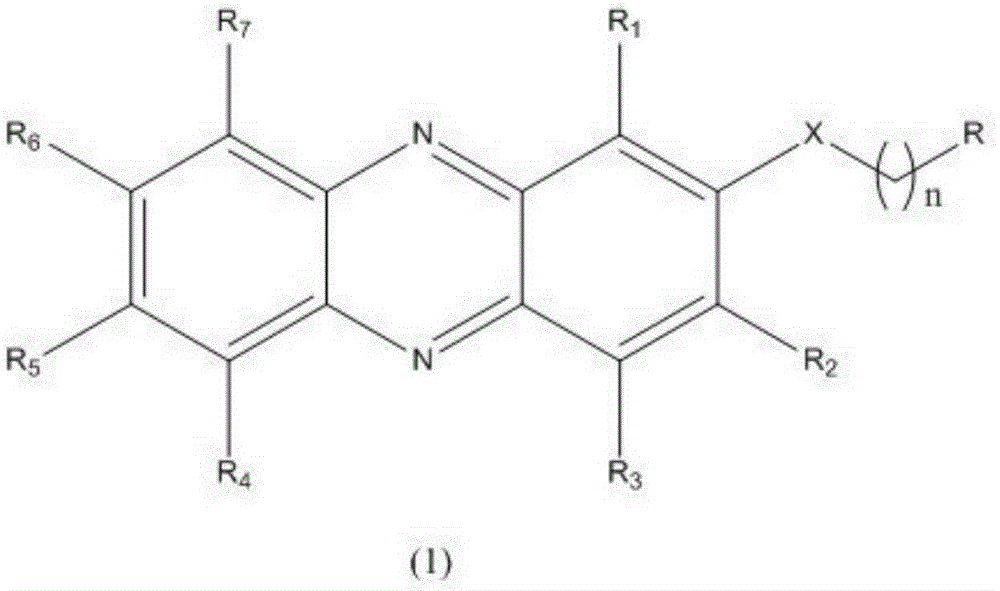
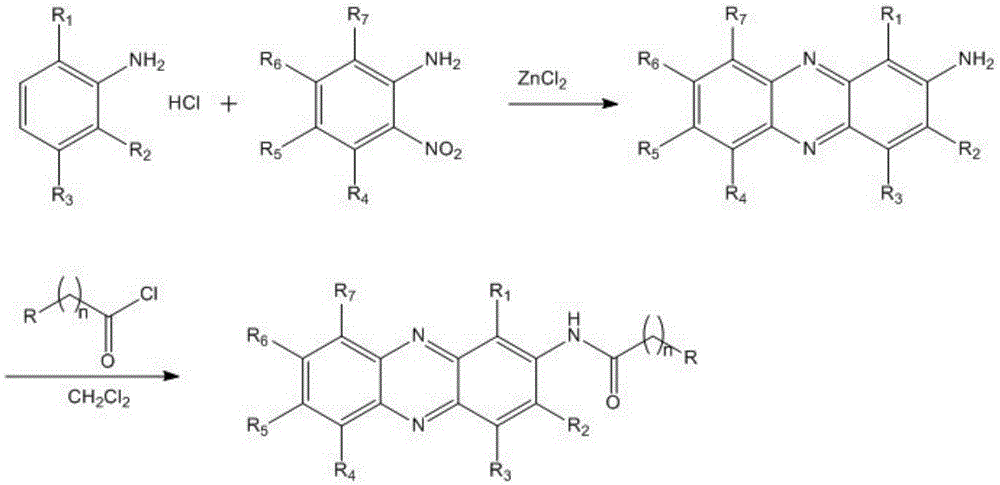
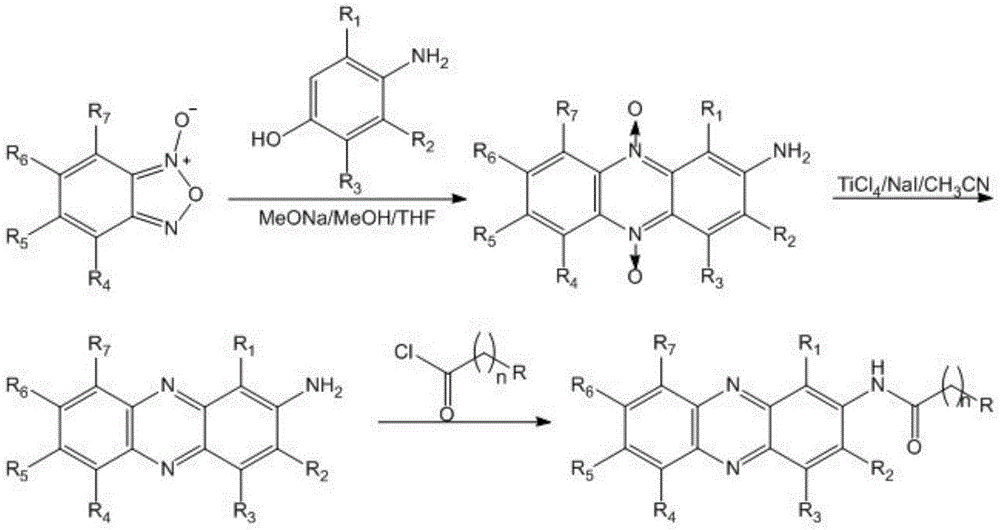
Novel phenazine substance, preparation method and application thereof
A technology of phenazine and substances, which is applied in the direction of medical preparations containing active ingredients, drug combinations, and pharmaceutical formulas, can solve the problems of low activity, poor selectivity, and high toxicity of anticancer drugs, and achieve significant anticancer activity and selectivity. Sex enhancement, less toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of 2-chloroacetamide-phenazine:
[0043]
[0044] Step 1: Synthesis of intermediate 2-amino-phenazine:
[0045]
[0046]Put 1.3g (0.01mol) of aniline hydrochloride, 1.4g (0.01mol) of o-nitroaniline and 4g (0.03mol) of zinc chloride in a reaction kettle, stir and react at 180-185°C for 30min, and start to react naturally after the reaction is over. Cool, cool to 100°C and extract twice with 20ml of ethanol under reflux, each extraction for 30min, then neutralize with saturated aqueous sodium carbonate solution, a solid precipitates, filter to take the filter cake, wash with dilute hydrochloric acid and methanol in turn, and vacuum dry to obtain Crude magenta solid 0.9g, yield 46.2%. mp:285-286℃(DEC); Infrared (KBr,cm -1 ):3307, 3190, 2915, 1640, 1598, 1512, 1474, 1456, 1335, 1236, 1130, 827, 755; 1 H-NMR (DMSO-d 6, 300MHz) δ: 8.05(m,2H,Ar-H), 7.89(m,2H,Ar-H), 7.79(dd,1H,Ar-H), 7.63(d,1H,Ar-H), 7.28( dd,1H,Ar-H), 5.25(s,2H,NH 2 ). 13 C-NMR (DMSO-d ...
Embodiment 2
[0051] Synthesis of N-[chloromethylamine]phenazine-2-carboxamide:
[0052]
[0053] Step 1: Synthesis of intermediate phenazine-2-carboxylic acid:
[0054]
[0055] Put 1.4g (0.01mol) of o-nitroaniline, 1.4g (0.01mol) of methyl benzoate and 4g (0.03mol) of catalyst zinc chloride in a reaction kettle and stir at 190-200°C for 30 minutes. After the reaction, start Natural cooling, cooling to 100°C, adding excess sodium hydroxide aqueous solution to hydrolyze it into phenazine-2-carboxylic acid, and forming a salt with sodium hydroxide, filtering to get the filtrate, adding excess hydrochloric acid aqueous solution to precipitate, separate and purify, and obtain the crude product White solid 0.7g, yield 30%. mp>300℃(DEC); 1 H-NMR (DMSO-d 6 ,300MHz) δ: 12.73(s,1H,OH), 9.44(d,1H,Ar-H), 9.05(d,1H,Ar-H), 8.38(d,1H,Ar-H), 8.27(m , 2H, Ar-H), 8.12 (m, 2H, Ar-H). 13 C-NMR (DMSO-d 6 ,125MHz) δ: 166.4(CO), 144.1(Ar-C), 142.4(Ar-C), 142.2(Ar-C), 142.1(Ar-C), 139.7(Ar-C), 132.3(...
Embodiment 3
[0060] Synthesis of 2-chloroacetamide-4-methyl-phenazine:
[0061]
[0062] Step 1: Synthesis of intermediate 2-amino-4-methyl-phenazine:
[0063]
[0064] Put 1.4g (0.01mol) of 3-methylaniline hydrochloride, 1.4g (0.01mol) of o-nitroaniline and 4g (0.03mol) of zinc chloride in a reaction kettle, and stir and react at 180-185°C for 30min After the reaction, start to cool naturally, cool to 100°C and use 20ml ethanol to reflux and extract twice for 30min, then neutralize with saturated aqueous sodium carbonate solution, solids are precipitated, filter to get the filter cake, and after treatment, 0.8g of crude brown solid is obtained. rate 38.3%. mp>300℃(DEC); 1 H-NMR (DMSO-d 6 ,300MHz)δ:8.33(m,2H,Ar-H), 8.18(m,2H,Ar-H), 7.81(d,1H,Ar-H), 7.47(d,1H,Ar-H), 5.32 (s,2H,NH 2 ), 2.32(s,3H,CH 3 ). 13 C-NMR (DMSO-d 6 ,125MHz) δ: 146.7(Ar-C), 144.2(Ar-C), 143.1(Ar-C), 142.3(Ar-C), 142.2(Ar-C), 138.0(Ar-C), 136.1(Ar -C), 129.7(Ar-C), 129.6(Ar-C), 129.3(Ar-C), 129.2(Ar-C), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com