A trans-structured lactone ring-containing nematocide and its preparation method and use
A technology of nematicides and lactone rings, applied in the field of nematicides containing lactone rings and its preparation, can solve the problems of high toxicity, phytotoxicity of crops, affecting use, etc., and achieve less harmfulness, less residue, Good insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
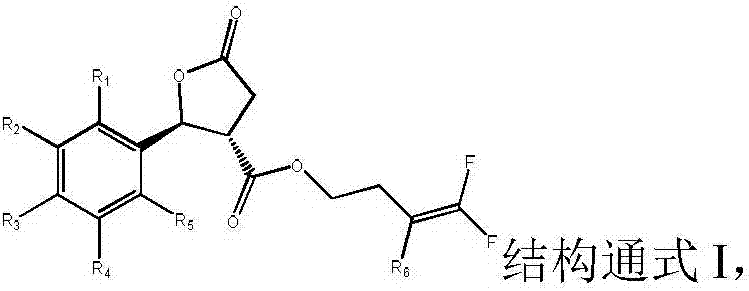
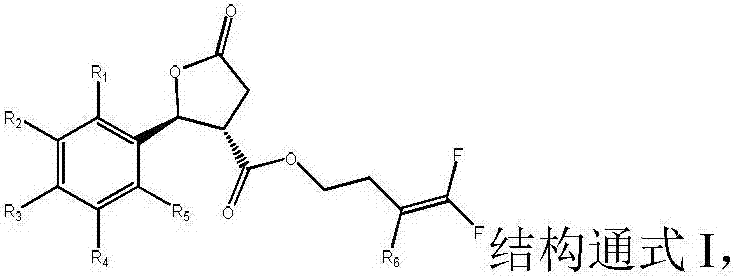
[0031] Preferably, a trans-structured lactone ring-containing nematicide, R 2 =R 4 = CF 3 when R 1 =R 3 =R 5 =H. The present invention also provides a preparation method of a trans-structured lactone ring-containing nematicide, comprising the following steps:
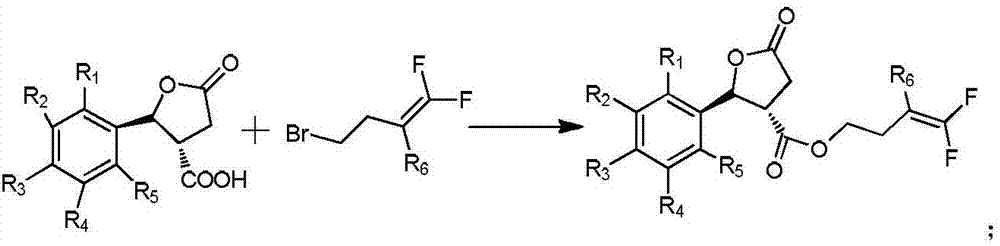
[0032] ① The acid with cis-trans racemization Add to sulfuric acid solution with a volume fraction of 40-70%, stir at 50-70°C for 3-5 hours, filter, add the filter cake to ethyl acetate and water, extract and collect the organic phase, dry the organic phase over magnesium sulfate, and evaporate the solvent , recrystallized from toluene and dried to give the trans-structured acid
[0033] ② will and the acid of the trans structure obtained in step 1. Add to the solvent, add the acid binding agent, stir and react at 20-30°C for 22-26 hours, under the vacuum of 0.08-0.10kPa, distill off the solvent, add dichloromethane and water and stir evenly, let stand to separate and remove water , under the vacuum degre...
Embodiment 1
[0052] The compound of serial number 2 in the preparation table 1 comprises the following steps:
[0053] ①Add 1mol of o-tolualdehyde, 0.8mol of succinic anhydride and 1mol of anhydrous zinc chloride into 300ml of dichloromethane, add 3mol of triethylamine dropwise at 0°C, and the reaction solution is obtained after the addition is completed, and the reaction solution is placed in Stir at 20°C for 10 hours, add hydrochloric acid therein to adjust the pH of the reaction solution to 2, add 200ml of ethyl acetate for extraction, collect the organic phase, add 200ml of toluene to the organic phase for recrystallization to obtain 5-oxo-2-(2-methyl phenyl)tetrahydrofuran-3-carboxylic acid;
[0054] ②Add 5-oxo-2-(2-methylphenyl)tetrahydrofuran-3-carboxylic acid obtained in step ① into 40% sulfuric acid solution, stir at 50°C for 3h, filter, and add the filter cake to ethyl acetate and water , extract and collect the organic phase, dry the organic phase over magnesium sulfate, evapor...
Embodiment 2
[0059] The compound of sequence number 26 in the preparation table 1 comprises the following steps:
[0060] ① Add 1mol of 4-fluoro-5-phenoxybenzaldehyde, 1.2mol of succinic anhydride and 3mol of anhydrous zinc chloride into 400ml of dichloromethane, add 5mol of triethylamine dropwise at 5°C, and the reaction solution is obtained after the dropwise addition is completed. Stir the reaction solution at 30°C for 20 hours, add hydrochloric acid to adjust the pH of the reaction solution to 2, add 250ml of ethyl acetate for extraction, collect the organic phase, add 250ml of toluene to the organic phase for recrystallization to obtain 5-oxo-2 -(2-(4-fluoro-5-phenoxy)phenyl)tetrahydrofuran-3-carboxylic acid;
[0061] ②Add 5-oxo-2-(2-(4-fluoro-5-phenoxy)phenyl)tetrahydrofuran-3-carboxylic acid obtained in step ① into 70% sulfuric acid solution, stir at 40°C for 5h, filter, Add the filter cake to ethyl acetate and water, extract and collect the organic phase, dry the organic phase ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com