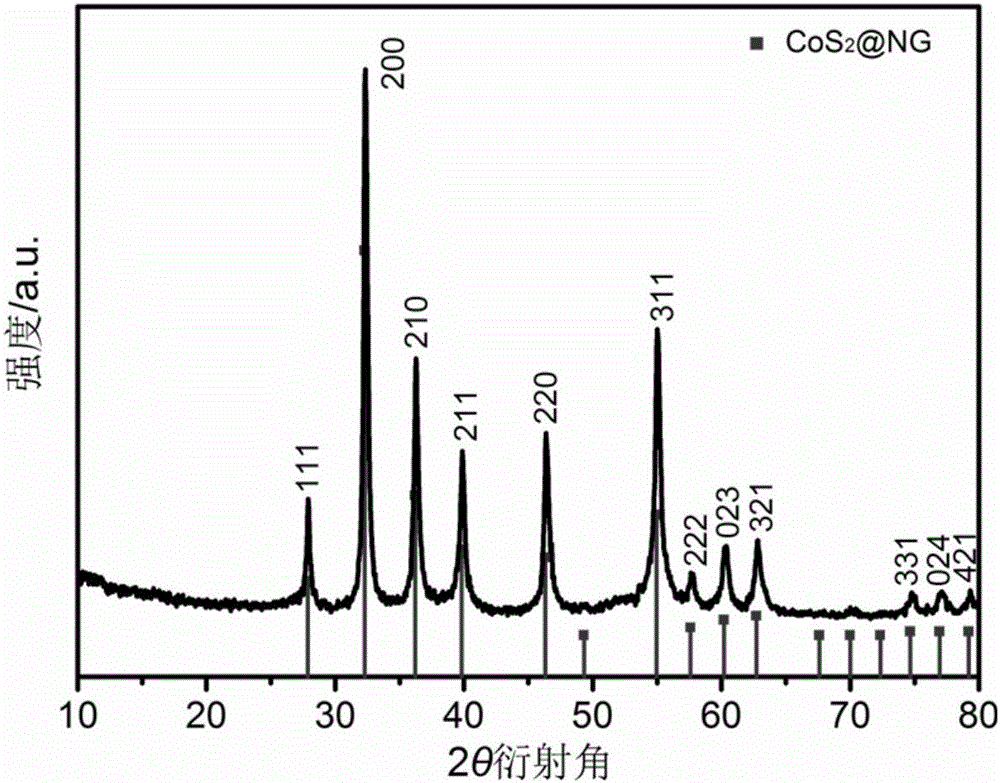
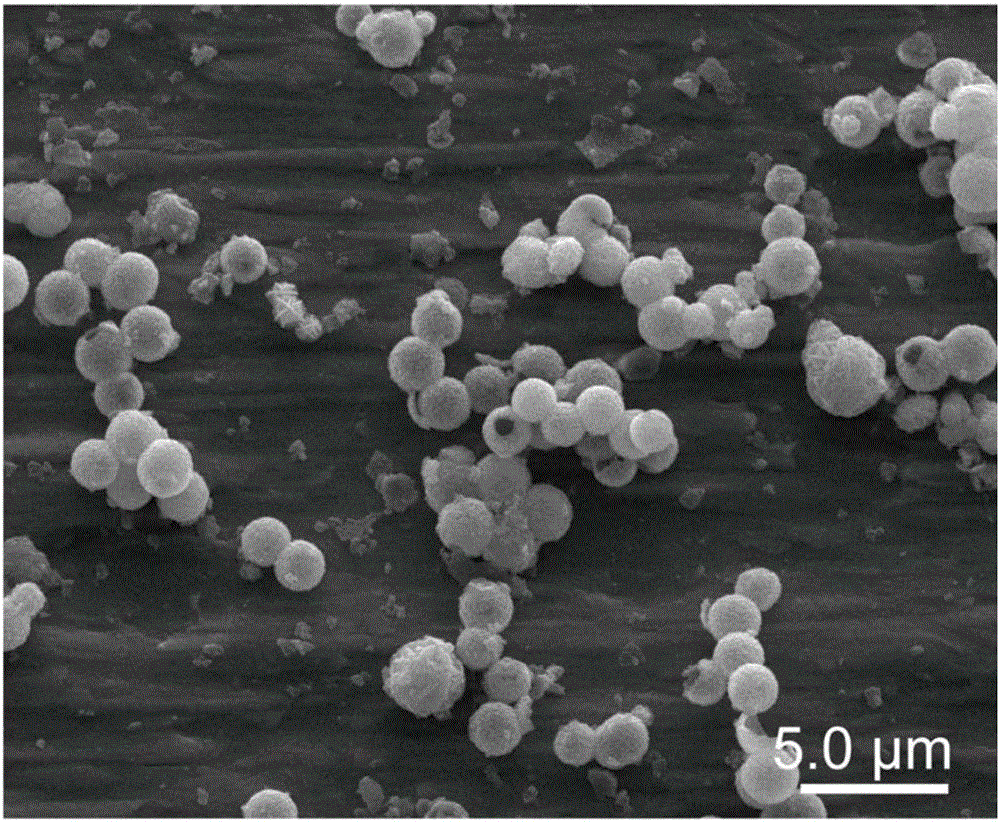
Core-shell CoS2@NG nanometer composite material, and preparation and application thereof
A nanocomposite material and nanoparticle technology, which is applied in the field of core-shell CoS2@NG nanocomposite materials and their preparation, can solve the problems of scarcity of precious metals and incapability of large-scale application, and achieve low cost, good hydrogen evolution electrocatalytic performance, and improved hydrogen evolution. Effect of hydrogen evolution catalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment includes the following steps:
[0045] (1) Graphene oxide was prepared by the modified Hummers method.
[0046] (2) 294.413mg CoCl 2 ·6H 2 O and 310.238 mg Na 2 S 2 o 3 ·5H 2 O was dissolved in a solution of 10 mL of deionized water and 30 mL of ethanol, and stirred for 3 h to obtain a uniformly mixed blue solution.
[0047] (3) Add the solution obtained in step 2 into the reaction kettle, and then seal it. A stainless steel autoclave was placed in an electric furnace and heated at 160 °C for 24 h, and then allowed to cool naturally to room temperature. Finally, the black precipitate was collected by centrifugation, washed thoroughly with deionized water and ethanol six times, and dried in a vacuum oven at 60 °C for 12 h.
[0048] (4) CoS obtained in step 3 2 The particles (0.5 g) were dispersed in 30 mL of toluene solution by sonication. After 1h, 0.5mL APS was added to the above solution and stirred for 24h to obtain APS-modified CoS 2 parti...
Embodiment 2
[0052] This embodiment includes the following steps:
[0053] (1) Graphene oxide was prepared by the modified Hummers method.
[0054] (2) 280mg CoCl 2 ·6H 2 O and 290 mg Na 2 S 2 o 3 ·5H 2 O was dissolved in a solution of 20mL deionized water and 40mL ethanol, and stirred for 3h to obtain a uniformly mixed blue solution.
[0055] (3) Add the solution obtained in step 2 into the reaction kettle, and then seal it. A stainless steel autoclave was placed in an electric furnace and heated at 140° C. for 26 h, and then allowed to cool naturally to room temperature. Finally, the black precipitate was collected by centrifugation, washed thoroughly with deionized water and ethanol six times, and dried in a vacuum oven at 60 °C for 12 h.
[0056] (4) CoS obtained in step 3 2 The particles (0.6 g) were dispersed in 50 mL of toluene solution by sonication. After 1h, 0.6mL APS was added to the above solution and stirred for 24h to obtain APS-modified CoS 2 particles. Then, GO ...
Embodiment 3
[0059] This embodiment includes the following steps:
[0060] (1) Graphene oxide was prepared by the modified Hummers method.
[0061] (2) 310mg CoCl 2 ·6H 2 O and 320 mg Na 2 S 2 o 3 ·5H 2 O was dissolved in a solution of 30mL deionized water and 50mL ethanol, and stirred for 3h to obtain a uniformly mixed blue solution.
[0062] (3) Add the solution obtained in step 2 into the reaction kettle, and then seal it. A stainless steel autoclave was placed in an electric furnace and heated at 180° C. for 18 h, and then allowed to cool naturally to room temperature. Finally, the black precipitate was collected by centrifugation, washed thoroughly with deionized water and ethanol six times, and dried in a vacuum oven at 60 °C for 12 h.
[0063] (4) CoS obtained in step 3 2 The particles (0.2 g) were dispersed in 40 mL of toluene solution by sonication. After 1h, 0.2mL APS was added to the above solution and stirred for 24h to obtain APS-modified CoS 2 particles. Then, GO ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com