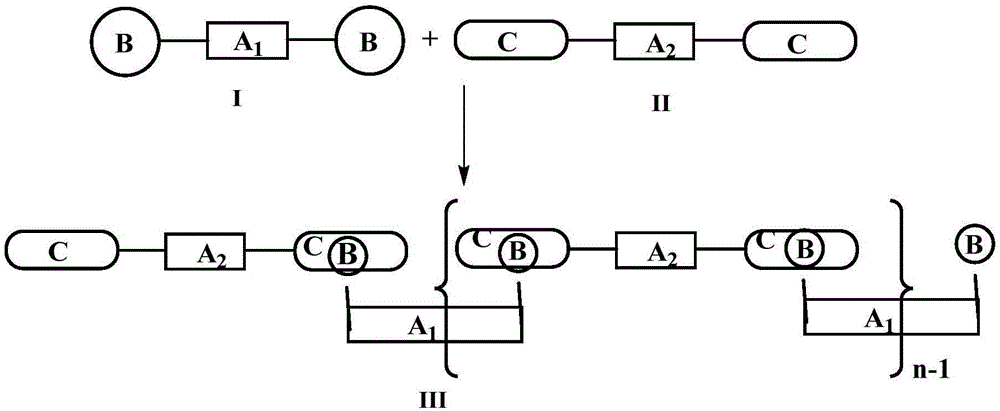
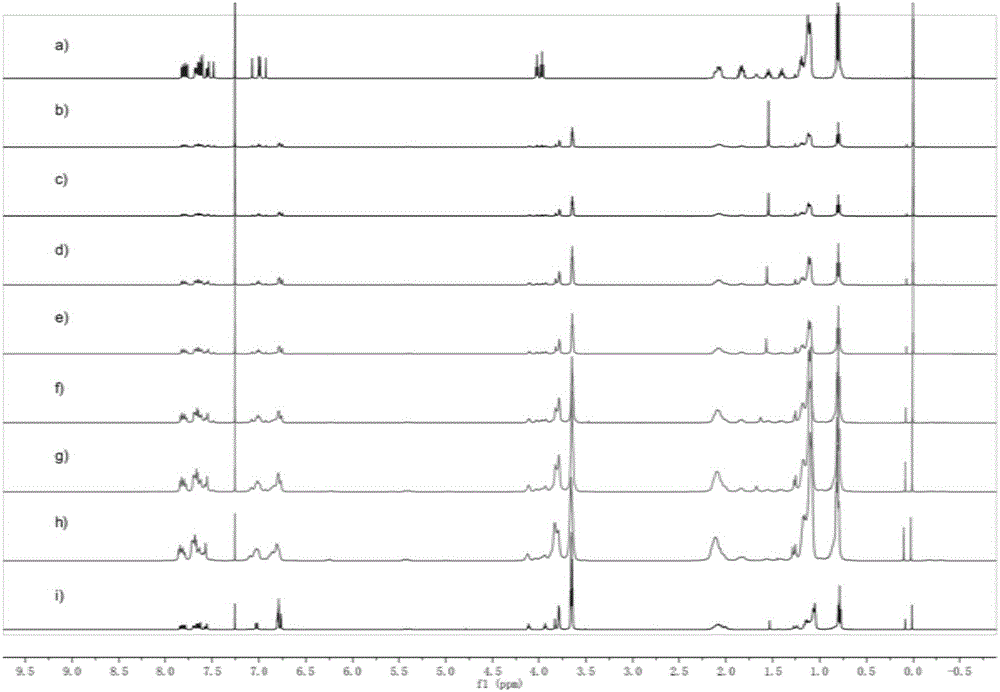
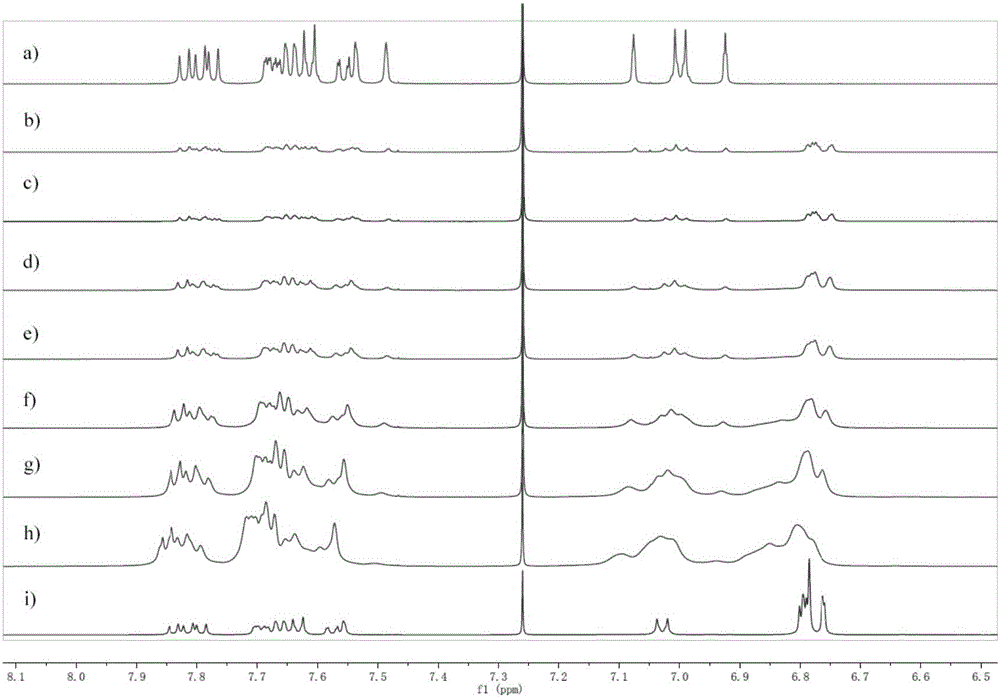
Pillararene-based supramolecular polymer photoelectric material, preparation method therefor and application of supramolecular polymer photoelectric material
A supramolecular polymer and optoelectronic material technology, which is applied in the field of pillar aromatic-based supramolecular polymer optoelectronic materials and their preparation, can solve the problems that the supramolecular polymer materials have not attracted attention, and achieve the effect of great commercial prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Pillararene monomer 7,7"-bis(pyra[5]arene)-2,2':7',2"-tris(9,9-dioctylfluorene)(I), 1,1'-{ [2,2':7',2"-tris(9,9-dioctylfluorene)]-bis(1,4-phenyl)}bis(oxy)bis(hexane-6,1diyl) Preparation of bis(1H-imidazole)(II)
[0046] The synthetic route is as follows:
[0047] Preparation of pillar arene monomer 7,7"-bis(pillar[5]arene)-2,2':7',2"-tri(9,9-dioctylfluorene) (I):
[0048]
[0049] Add monofunctionalized 1-bromooctyloxy-4-methoxy-pillar[5]arene (1.76g, 2mmol), cesium carbonate (3.94g, 12mmol), and add 30mL of N,N-dimethyl base formamide. Heated to reflux for 24h. The reaction was left to room temperature, dichloromethane was added, extracted, the organic layer was washed with water, and the organic phase was combined. This step was repeated three times. After drying over magnesium sulfate, the solvent was removed. It was eluted with a mixed solvent of petroleum ether / dichloromethane with a volume ratio of 1:1 as the eluent, and then separated by silica gel chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com