Preparation method of Alpha-amylase
An amylase and gene technology, applied in the field of α-amylase preparation, can solve the problems of difficulty in obtaining pure products and cumbersome purification steps of α-amylase, and achieve the effect of good safety and simple purification operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] Experimental Example 1 Preparation of α-amylase.
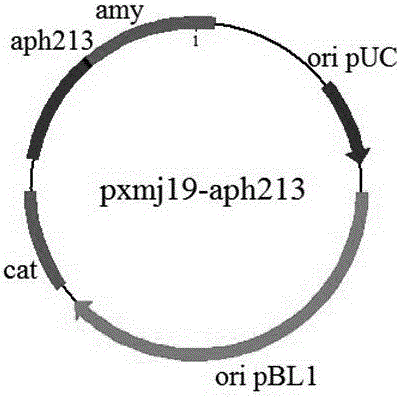
[0023] (1) Preparation of vector pxmj19-aph213.
[0024] The structure of the vector pxmj19-aph213 is as figure 1 As shown, its construction process is as follows:
[0025] The aph213 gene was amplified from the vector xk99e vector with primers aph213F and aph213R, and the primers used were as follows:
[0026] aph213F: ccggatatcagcttcacgctgccgcaagcac
[0027] aph213R: ccgaagcttaattctgtttcctgtgtgaaattg
[0028] The PCR conditions are as follows: 95°C for 4min; 35 cycles of 95°C for 30s, 62°C for 30s, and 72°C for 1min; 72°C for 7min.
[0029] The design of the above primers makes the aph213 gene amplification process form an EcoRV cut point upstream of the aph213 gene and a HindIII cut point downstream. Restriction digestion, T4 DNA ligase, and connection to the vector pxmj-19 that has also been digested by EcoRV and HindIII, to obtain the vector pxmj19-aph213.
[0030] The amplification process of the vector pxmj...
Embodiment 2
[0052] Example 2 Identification of alpha-amylases.
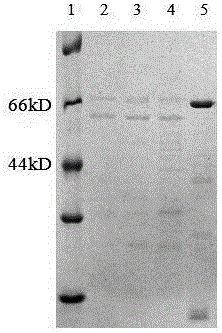
[0053] (1) SDS-PAGE identification.
[0054] Centrifuge the fermentation broth, take 10ul of the supernatant for SDS-PAGE, to detect the band size of the protein and the purity of the purification, the results are as follows figure 2 As shown, the results show that compared with the empty vector, the supernatant band with the recombinant α-amylase vector will have an extra band of about 68kD, and the purification result will also have a single band, such as figure 2 As shown, lane 5 is the purified α-amylase, lane 1 is the protein marker, lane 2 is the supernatant of Corynebacterium glutamicum, and lane 3 is the supernatant containing α-amylase that has not been adsorbed after being purified by a nickel column. Flow-through, lane 4 is the supernatant containing α-amylase.
[0055] (2) Identification by DNS method.
[0056] In the experimental group, add 50ul enzyme solution to 250ul pH5.0 citrate disodium hydrogen phosp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com