Minimal saponin analogues, synthesis and use thereof
A technology of drugs and compounds, applied in the field of adjuvants derived from triterpene glycosides and saponins, which can solve problems such as limited access to natural sources, hindering the rational development of analogues, and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Example 1: Preliminary evaluation of iodinated saponins 6 and 8
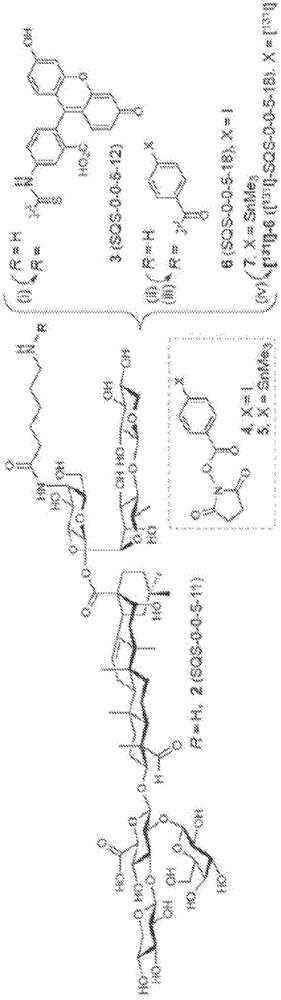
[0173] Radioactive iodine ( 131 I) Introduced into the QS-21 saponin backbone to achieve biodistribution studies in vivo. Synthesis of non-radiolabeled aryl iodide 6 (SQS-0-0-5-18) via acylation of amine 2 (SQS-0-0-5-11) ( Figure 1b ). Because no in vitro model exists to assess adjuvant activity, the biological evaluation of aryl iodide 6 was performed in a preclinical mouse vaccination model involving a multi-antigen formulation comprising the immunogenic peptide MUC1 (prostate and Breast cancer antigen, aglycosylated tandem repeat), which is conjugated to a highly immunogenic KLH carrier protein (MUC1-KLH) and OVA, a reliable immunogen that induces antibody and T cell responses in mice. Antibody responses to each of the three antigens co-administered with the adjuvants of interest were determined by ELISA.
[0174] Antibody titers induced by aryl iodide saponin 6 (SQS-0-0-5-18) were comparable to N...
Embodiment 2
[0176] Embodiment 2: radioactive iodinated saponins [ 131 I] -6 and[ 131 I] -8 Biodistribution of
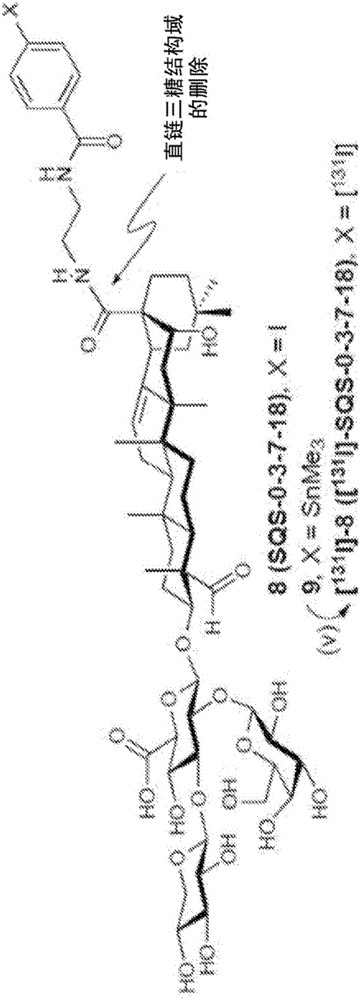
[0177] For biodistribution studies, radiolabeled aryl iodide saponins were synthesized by aryl tin-halide exchange of trimethylstannane 7[ 131 I] -6 ([ 131 I]-SQS-0-0-5-18)( Figure 1b ). Radioiodinated [ 131 I] -8 ([ 131 I]-SQS-0-3-7-18) as a negative control ( Figure 1c ).
[0178] To identify tissues and organs capable of playing a role in the saponin mechanism of action, the active adjuvant [ 131 I] -6 The acute in vivo biodistribution and attenuated adjuvant in mice co-administered with OVA[ 131 I] -8 Compare. and[ 131 I] -8 Compared with [ 131 I] -6 Treatment resulted in ( Figure 2a ) significantly higher recovery of radioactivity, which was retained at 72 and 96 hours post-injection (Figure 8). Conversely, radioactivity in other tissues where large fold differences were initially observed (muscle, bone, skin) decreased rapidly at later time points....
Embodiment 3
[0183] Example 3: Truncated saponins lacking branched trisaccharide domains (16)
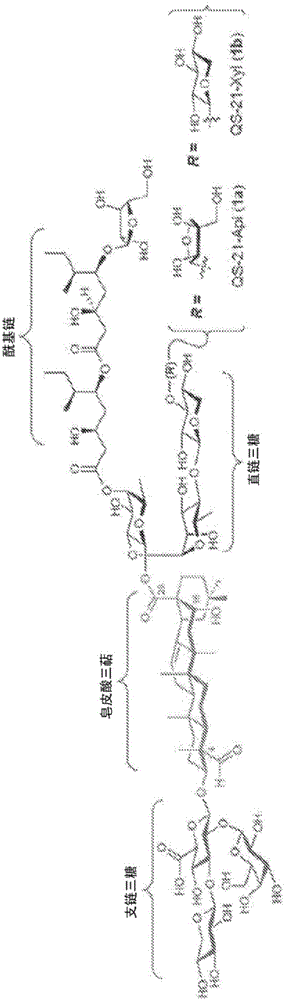
[0184] The essential triterpene core is selectively silylated at the hydroxyl group (TESOTf, 2,6-lutidine) to provide the protected triterpene with free C28-carboxylic acid. β-selective Schmidt glycosylation reaction with trisaccharide trichloroacetimide donor 12 (BF 3 ·OEt 2 ) (Chea, E.K. et al. Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J. Am. Chem. Soc. 134, 13448-13457 (2012)), followed by reduction of azide (PhSeH), The corresponding glucosyl esters are obtained. The amine and 6-((tert-butoxycarbonyl)-amino)caproic acid (14) (EtOCOCl, Et 3 N) acylation reaction, and subsequent hydrogenolysis (H 2 , Pd / C) and acid hydrolysis (TFA / H 2 O) Global deprotection, resulting in a fully deprotected saponin with a free amine at the end of the acyl chain domain. Late acylation of this amine with succinimidyl esters 4 or 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com