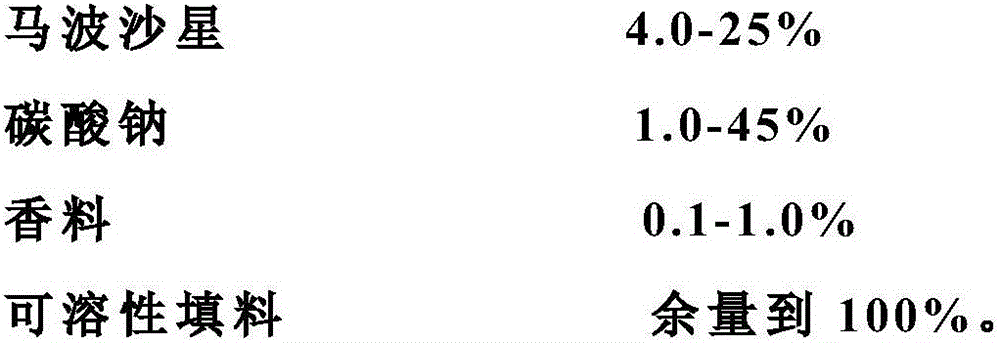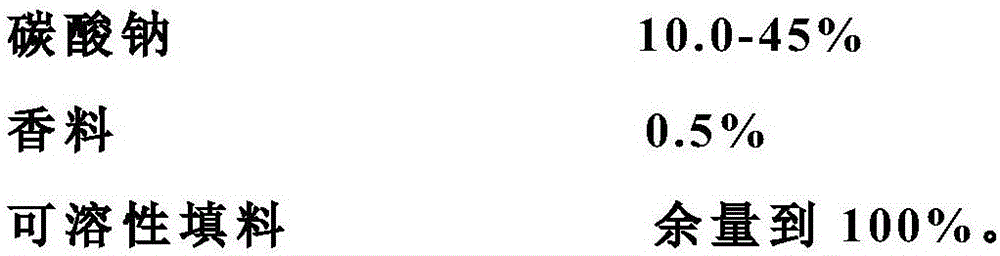Marbofloxacin soluble pulvis
A soluble powder, marbofloxacin technology, applied in the field of biomedicine, can solve the problems of high heat sensitivity, inconvenient transportation, easy decomposition and deterioration, etc., and achieve the effect of good water solubility, convenient transportation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Marbofloxacin soluble powder, which is mainly based on marbofloxacin and sodium carbonate (containing 10% marbofloxacin, 45% sodium carbonate), with an appropriate amount of spices, soluble correctives and soluble fillers. It is prepared by conventional methods such as drying, weighing, crushing, stirring, mixing, inspection, measurement, and packaging.
[0024] The formula is:
[0025]
[0026] Specific production method:
[0027] (1) Dry the above raw materials to make the water content less than 5%.
[0028] (2) Weigh, fully pulverize the raw materials of the above components, and use 60 to 80 mesh sieve for sieving treatment;
[0029] (3) Pour the above-mentioned raw materials after sieving into the V-type mixer, and stir them for 25 minutes to mix evenly;
[0030] (4) Sampling and testing to check the percentage of raw material content, whether the fineness of powder meets the standard, whether the moisture content is qualified, etc.;
[0031] (5) Measure and weigh, pack into...
Embodiment 2
[0033] The marbofloxacin soluble powder in this embodiment contains 10% marbofloxacin, 45% sodium carbonate, 1% musk flavor, 30% soluble corrective agent, and 14% starch. Dry 10 grams of marbofloxacin, 45 grams of sodium carbonate, 1 gram of musk flavor, 30 grams of sucrose, and 14 grams of starch to make the water content less than 5%, weigh it, fully crush the raw materials of the above components, and use 60 to 80 mesh sieve for sieving treatment. Pour the sieved raw materials and auxiliary materials into the V-type mixer, fully stir for 25 minutes, and mix well.
[0034] Sampling and testing to check the percentage of raw materials, whether the fineness of the powder meets the standard, whether the water content is qualified, etc., measure and weigh, pack into a bag, and pack 5 grams, 10 grams or 100 grams of the above powder into each bag to obtain Mabosha Star soluble powder.
Embodiment 3
[0036] The marbofloxacin soluble powder in this embodiment contains 20% marbofloxacin, 10% sodium carbonate, 1% musk flavor, 25% lactose, and 44% glucose. Dry 20 grams of marbofloxacin, 15 grams of sodium carbonate, 1 gram of musk flavor, 25 grams of lactose, and 44 grams of glucose to make the water content less than 5%. Weigh it. The raw materials of the above components are fully crushed and used 60 to 80 mesh sieve is sieved. Pour the sieved raw materials and auxiliary materials into the V-type mixer, fully stir for 25 minutes, and mix evenly.
[0037] Sampling and testing to check the percentage of raw materials, whether the fineness of the powder meets the standard, whether the water content is qualified, etc., weigh it, pack it into a bag, and fill each bag with 5 g, 10 g or 100 g of the above powder to obtain Mabosha Star soluble powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com