Method for preparing 5-hydroxymethylfurfural through fructose dehydration catalyzing
A technology of hydroxymethyl furfural and fructose, applied in the field of preparing 5-hydroxymethyl furfural, can solve problems such as unreported, and achieve the effects of low cost, high activity and selectivity, easy separation, recovery and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
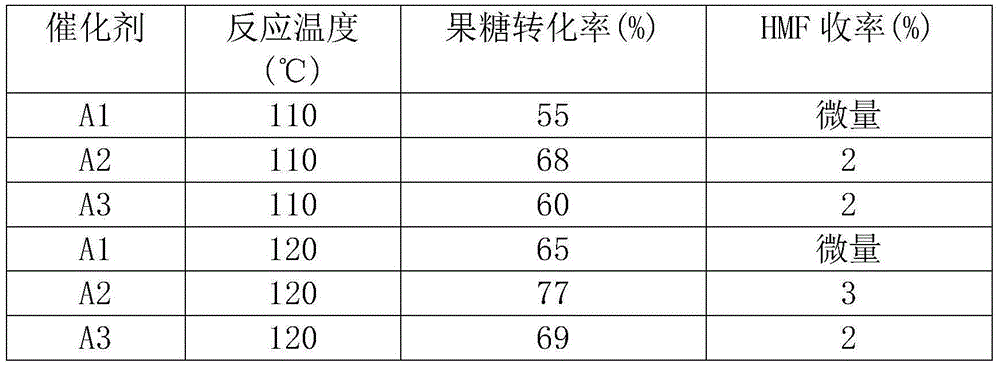
[0025] Embodiment 1 Material A1 (B 2 o 3 -CeO 2 -1:4) preparation
[0026] Mix and dissolve 18.9g of cerium nitrate hexahydrate and 0.676g of boric acid in 50mL of ethanol, stir at room temperature until completely dissolved, remove the ethanol by rotary evaporation to obtain a viscous colloidal colorless substance, and use liquid nitrogen to freeze and quickly dry to obtain a white solid substance. Then the obtained solid substance was ground evenly and placed in a roasting tube, the air flow rate was set to 50mL / min, the heating rate was 5°C / min, the temperature was raised to 450°C and kept for 4h, and finally the yellow solid substance A1 (B 2 o 3 -CeO 2 -1:4).
Embodiment 2
[0027] Embodiment 2 Material A2 (B 2 o 3 -CeO 2 -1:4) preparation
[0028] The preparation method of material A2 is the same as that of material A1, except that the final calcination temperature of the material is different. Mix and dissolve 18.9g of cerium nitrate hexahydrate and 0.68g of boric acid in 50mL of ethanol, stir at room temperature until completely dissolved, remove the ethanol by rotary evaporation to obtain a viscous colloidal colorless substance, and use liquid nitrogen to freeze and quickly dry to obtain a white solid substance. Then the obtained solid substance was ground evenly and placed in a roasting tube, the air flow rate was set to 50mL / min, the heating rate was 5°C / min, the temperature was raised to 550°C and kept for 4h, and finally the yellow solid substance A2 (B 2 o 3 -CeO 2 -1:4).
Embodiment 3
[0029] Embodiment 3 Material A3 (B 2 o 3 -CeO 2 -1:4) preparation
[0030] The preparation method of material A3 is the same as that of materials A1 and A2, except that the final firing temperature of the materials is different. Mix and dissolve 18.9g of cerium nitrate hexahydrate and 0.68g of boric acid in 50mL of ethanol, stir at room temperature until completely dissolved, remove the ethanol by rotary evaporation to obtain a viscous colloidal colorless substance, and use liquid nitrogen to freeze and quickly dry to obtain a white solid substance. Then the obtained solid substance was ground evenly and placed in a roasting tube, the air flow rate was set to 50mL / min, the heating rate was 5°C / min, the temperature was raised to 600°C and kept for 4h, and the yellow solid substance A3(B 2 o 3 -CeO 2 -1:4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com