Method for secretory expression of super-folded green fluorescent protein mediated heterologous protein in escherichia coli
A technology of green fluorescent protein and heterologous protein, which is applied in the field of extracellular secretion and expression of heterologous protein mediated by superfolded green fluorescent protein in Escherichia coli, which can solve the problems of complex operation, complicated operation process and low yield of target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
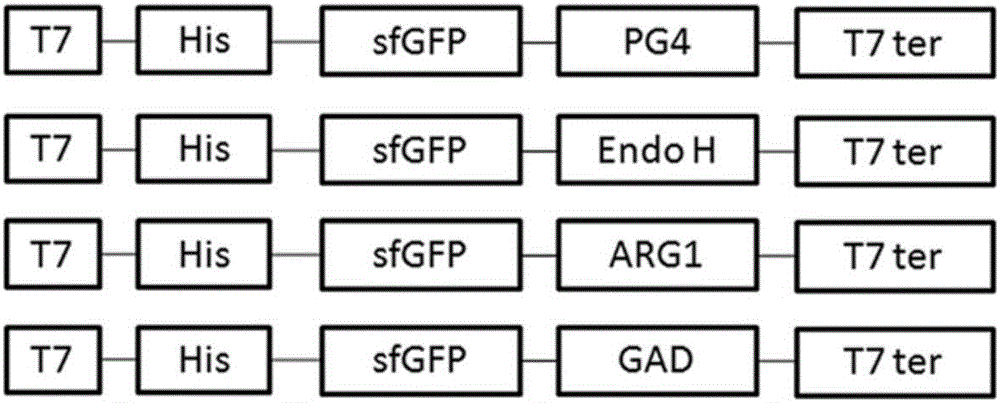
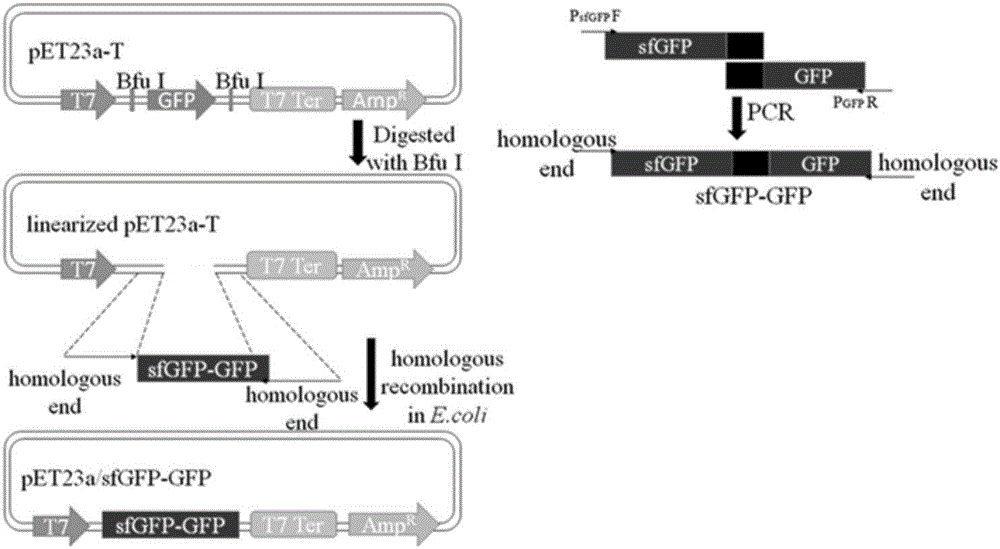
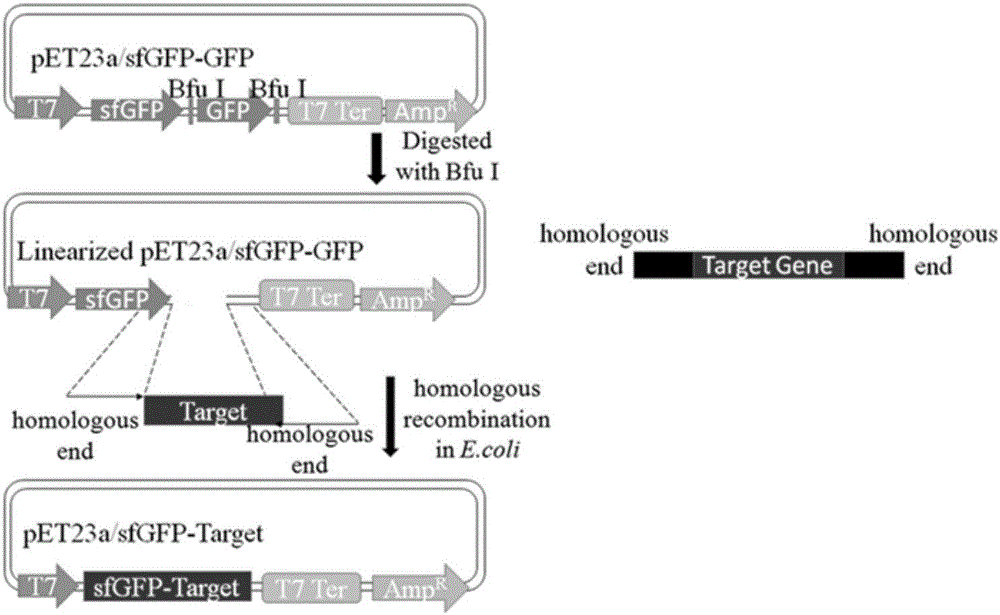
[0075] Superfolded green fluorescent protein (sfGFP) mediates the extracellular secretion and expression of toxic protein (antimicrobial peptide PG4) in Escherichia coli RosettaBlue. First, the constructed recombinant plasmid pET23a / sfGFP-PG4 was transformed into Escherichia coli competent cell Rosetta Blue strain, and cultured overnight at 37°C to obtain the recombinant strain. Next, pick a single colony and inoculate it in 100 mL of LB liquid medium (the concentration of ampicillin is 50 μg / mL), culture it with shaking at 37°C, the OD value is between 0.5 and 0.6, add IPTG, the final concentration is 1mM, shake at 37°C Incubate for 8 hours. After the cultivation is completed, the bacterial cells and the medium supernatant are collected by centrifugation at 12000 RPM at 4°C. SDS-PAGE detection and analysis of fusion protein secretion and expression.
Embodiment 2
[0077]Superfolded green fluorescent protein (sfGFP)-mediated enzyme (β-N-acetylglucosaminidase H, Endo H) was secreted and expressed extracellularly in Escherichia coli Rosetta Blue. First, the constructed recombinant plasmid pET23a / sfGFP-Endo H was transformed into Escherichia coli competent cell Rosetta Blue strain, and cultured overnight at 37°C to obtain the recombinant strain. Next, pick a single colony and inoculate it in 100 mL of LB liquid medium (the concentration of ampicillin is 50 μg / mL), culture it with shaking at 37°C, the OD value is between 0.5 and 0.6, add IPTG, the final concentration is 1mM, shake at 37°C Incubate for 8 hours. After the cultivation is completed, the bacterial cells and the medium supernatant are collected by centrifugation at 12000 RPM at 4°C. The samples were processed as follows: ①SDS-PAGE detection and analysis of the secretion and expression of the fusion protein; ②Referring to the detection method in the content of the invention, the e...
Embodiment 3
[0079] Superfolded green fluorescent protein (sfGFP) mediates the extracellular secretion expression of homotrimeric protease (human arginase-1, ARG1) in Escherichia coli Rosetta Blue. First, the constructed recombinant plasmid pET23a / sfGFP-ARG1 was transformed into Escherichia coli competent cell Rosetta Blue strain, and cultured overnight at 37°C to obtain the recombinant strain. Next, pick a single colony and inoculate it in 100 mL of LB liquid medium (the concentration of ampicillin is 50 μg / mL), culture it with shaking at 37°C, the OD value is between 0.5 and 0.6, add IPTG, the final concentration is 1mM, shake at 37°C Incubate for 8 hours. After the cultivation is completed, the bacterial cells and the medium supernatant are collected by centrifugation at 12000 RPM at 4°C. The samples are processed as follows: ① SDS-PAGE detection and analysis of the secretion and expression of the fusion protein; ② referring to the detection method in the content of the invention, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com