Pharmaceutical composition containing ibrutinib
A technology of ibrutinib and composition, applied in the field of pharmaceutical preparation, can solve the problems of propylene glycol dosage limitation, safety problems, etc., and achieve the effects of excellent emulsification, good low-temperature washing performance and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
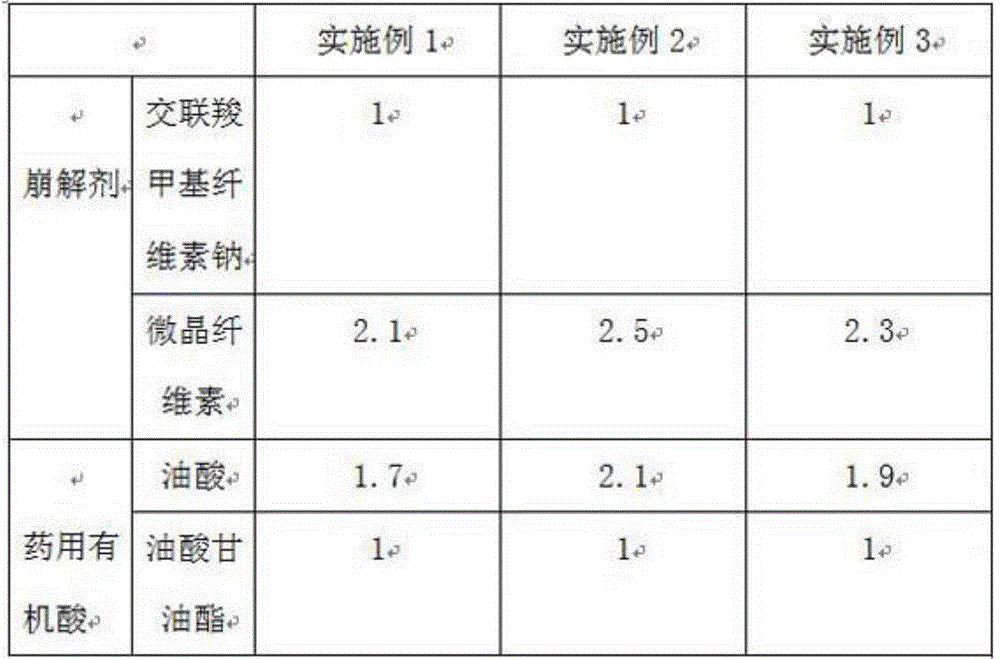
[0018] This embodiment contains the pharmaceutical composition of ibrutinib, which comprises the following components in parts by weight: 13 parts of ibrutinib, 50 parts of glycerol formal solvent, 30 parts of solubilizer, N-methylcarbamoyl chloride 12 parts of filler, 10 parts of disintegrating agent and 26 parts of medicinal organic acid; among them, the solubilizer is selected from hydrophilic surfactant with HLB value of 15-18, and the hydrophilic surfactant is polyoxyethylene fatty alcohol ether; The disintegrant is a mixture of carboxymethylcellulose calcium and microcrystalline cellulose, with 3.23 parts of carboxymethylcellulose calcium and 6.77 parts of microcrystalline cellulose; the medicinal organic acid is a mixture of oleic acid and glyceryl oleate , and 16.37 parts of oleic acid, 9.63 parts of glyceryl oleate.
Embodiment 2
[0020] This embodiment contains the pharmaceutical composition of ibrutinib, which comprises the following components in parts by weight: 13 parts of ibrutinib, 50 parts of glycerol formal solvent, 30 parts of solubilizer, N-methylcarbamoyl chloride 12 parts of filler, 10 parts of disintegrating agent and 26 parts of medicinal organic acid; among them, the solubilizer is selected from hydrophilic surfactant with HLB value of 15-18, and the hydrophilic surfactant is polyoxyethylene fatty alcohol ether; The disintegrant is a mixture of carboxymethylcellulose calcium and microcrystalline cellulose, with 2.86 parts of carboxymethylcellulose calcium and 7.14 parts of microcrystalline cellulose; the medicinal organic acid is a mixture of oleic acid and glyceryl oleate , and 17.61 parts of oleic acid, 8.39 parts of glyceryl oleate.
Embodiment 3
[0022] This embodiment contains the pharmaceutical composition of ibrutinib, which comprises the following components in parts by weight: 13 parts of ibrutinib, 50 parts of glycerol formal solvent, 30 parts of solubilizer, N-methylcarbamoyl chloride 12 parts of filler, 10 parts of disintegrating agent and 26 parts of medicinal organic acid; among them, the solubilizer is selected from hydrophilic surfactant with HLB value of 15-18, and the hydrophilic surfactant is polyoxyethylene fatty alcohol ether; The disintegrating agent is composed of carboxymethylcellulose calcium and microcrystalline cellulose, with 3.03 parts of carboxymethylcellulose calcium and 6.97 parts of microcrystalline cellulose; the medicinal organic acid is a mixture of oleic acid and glyceryl oleate , and 17.03 parts of oleic acid, 8.97 parts of glyceryl oleate.
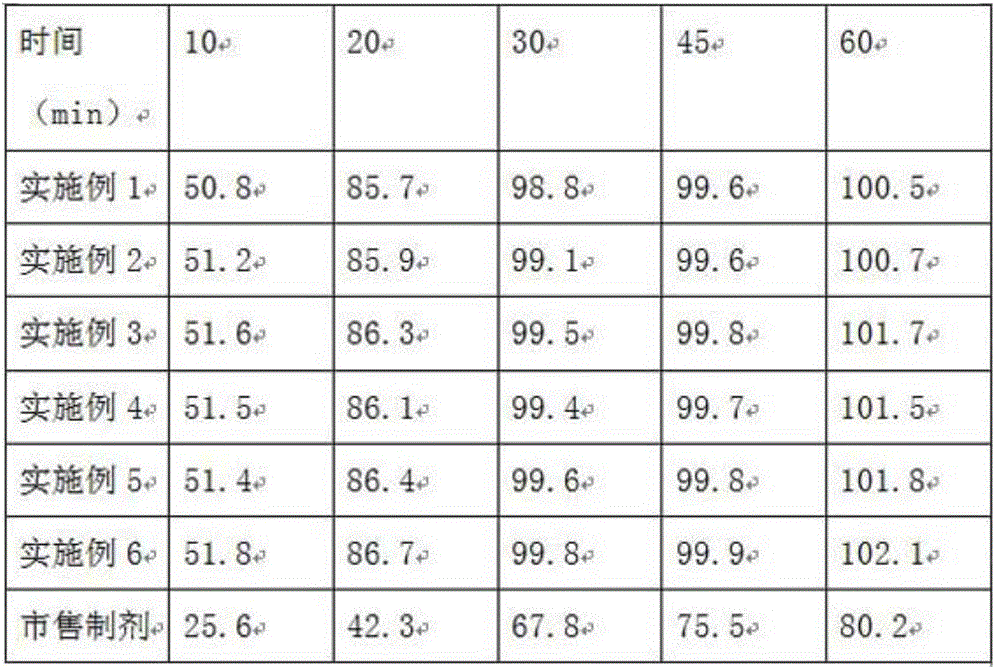
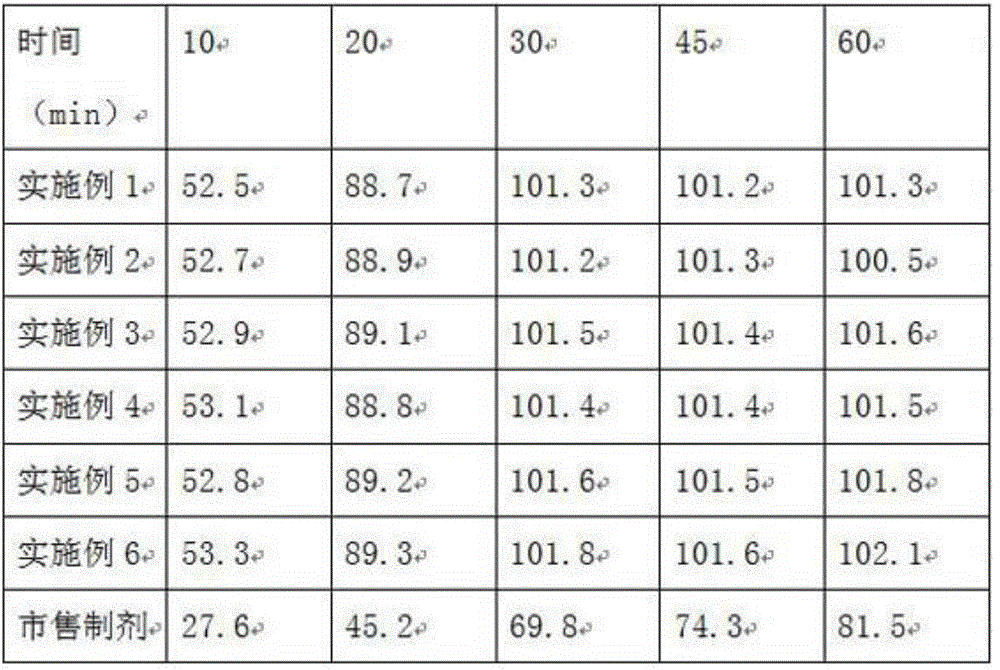
[0023] Table 1 is a comparison table of the mass ratio of oleic acid and glyceryl oleate in the disintegrant in Examples 1-3, croscarmellose sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com