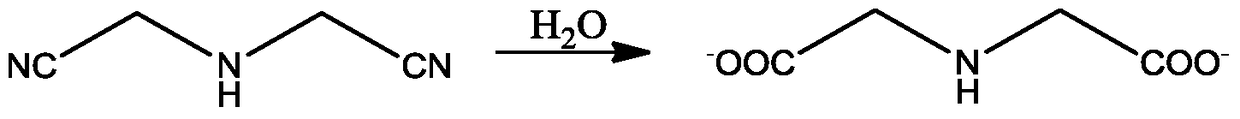A kind of preparation method of n1-(2-aminoethyl)-1,2-ethylenediamine
A technology based on ethylenediamine and aminoethyl, applied in the field of organic amine synthesis, can solve problems such as catalyst deactivation, three wastes, and environmental pollution that cannot be completely solved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The treated 5g of Raney cobalt and 0.075g of 5% Ru / C were pre-added into a 1L high-pressure stainless steel hydrogenation reactor, and then 500g of iminodiacetonitrile with a mass fraction of 5% iminodiacetonitrile was added. Hexacyclic solution, and then add 17mg of NaOH to the reaction kettle. Replace with 0.3Mpa nitrogen for 3 times, then replace with 0.3MPa hydrogen for 3 times, then raise the temperature to 85°C, add hydrogen and carbon monoxide mixed gas (H 2 : CO = 500: 1, volume ratio) to a pressure of 5 MPa, stirring to 1000 rpm. During the reaction process, the pressure of the mixer was maintained at 5MPa. At the same time, 20wt% formic acid was added dropwise when the amount of hydrogen absorbed by the reaction reached 10% of the theoretical value of hydrogen absorbed by the reaction, and 10% by weight of formic acid was added dropwise for every 10% increase in the amount of hydrogen absorbed later. The method for formic acid is that 25.5mg formic acid is ad...
Embodiment 2
[0069] Other conditions are the same as in Example 1, only the reaction temperature is changed to: 70°C, the reaction pressure is changed to 7.5MPa, the IDAN concentration is changed to 15%, the catalyst 1 quality is 5g, the catalyst 2 quality is 0.05g, and the auxiliary agent base is KOH , the quality is 37.5 mg, the acid added dropwise is glacial acetic acid, and the total mass is 61 mg; when the reaction starts, the volume ratio of feeding hydrogen and carbon monoxide gas mixture is H 2 : CO=1000:1, when the reaction reaches 25%, the system pressure is released to normal pressure, and the hydrogen is switched to high-purity hydrogen with a purity of 99.999%.
[0070] Catalyst application test results are as follows:
[0071] Apply times
Embodiment 3
[0073] Other conditions are the same as in Example 1, except that the reaction temperature is changed to 100° C., the reaction pressure is changed to 10 MPa, the IDAN concentration is changed to 25%, the mass of catalyst 1 is 5 g, and the mass of catalyst 2 is 0.025 g. The basic compound is KOH and the acidic compound is acetic acid. The quality of KOH is 66.5mg, and the quality of acetic acid is 133mg; When the reaction starts, the volume ratio of feeding hydrogen and carbon monoxide gas mixture is H 2 : CO=100:1, when the reaction reaches 30%, the system pressure is released to normal pressure, and the hydrogen is switched to high-purity hydrogen with a purity of 99.999%.
[0074] Catalyst application test results are as follows:
[0075] Apply times
[0076] 7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com