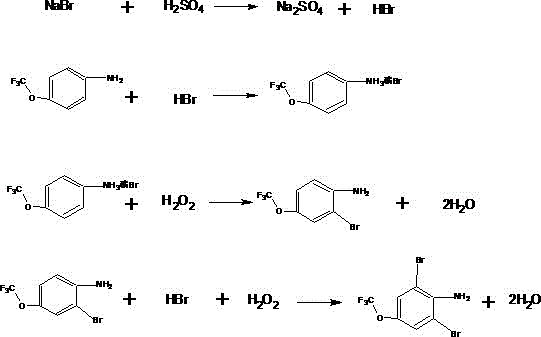Preparation method of thifluzamide key intermediate, namely 2,6-dibromo-4-(trifluoromethoxy)aniline
A technology of trifluoromethoxyaniline and trifluoromethoxy, which is applied in the synthesis field of thifuramide key intermediate-2,6-dibromo-4-aniline, can solve the problems of incomplete reaction and the like, and achieve the reaction The effect of rapid thoroughness, yield and purity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of 2,6-dibromo-4-(trifluoromethoxy)aniline, comprising the following steps:
[0031] Put 250g of water into a 1000mL three-necked bottle, start stirring and drop in 17.5 (0.175mol) sulfuric acid, then add 45g of potassium bromide (0.378moL) and 0.3g of tetrabutylammonium bromide, and then add 30g (99%, 0.168 mol) 4-trifluoromethoxyaniline, adjust the temperature to above 30°C, start to add 38g of 35% hydrogen peroxide dropwise, the temperature rises during the dropwise addition, when the temperature rises to 50°C, control the dropwise temperature at 50°C by controlling the cooling liquid Between ~55°C, keep the temperature until the drop is completed, keep the temperature for 3 hours after dropping, cool down to about 30°C, filter, and dry the filter cake to obtain white crystal 2,6-dibromo-4-trifluoromethoxyaniline 55.6 g, purity 99.8% (HPLC), yield 99%.
[0032] Suction filter the mother liquor and cool it down to 0°C, then filter again to obtain...
Embodiment 2
[0034] A preparation method of 2,6-dibromo-4-(trifluoromethoxy)aniline, comprising the following steps:
[0035] Put 310g of the mother liquor in which potassium sulfate crystals were filtered out in Example 1 into a 1000mL three-necked flask, start stirring and drop in 17.5 (0.175mol) sulfuric acid, then add 43.5g of potassium bromide (0.365mol) and 30g (99%, 0.168mol) 4-Trifluoromethoxyaniline, adjust the temperature above 30°C, start to add 38g (0.391mol) of 35% hydrogen peroxide dropwise, the temperature rises during the dropwise addition, when the temperature rises to 50°C, control the dropwise temperature by controlling the cooling liquid Keep the temperature between 50 and 55°C, keep the temperature for dripping, keep warm for 3.5 hours after dripping, cool down to about 30°C, filter, and dry the filter cake to obtain white crystal 2,6-dibromo-4-trifluoromethoxy Aniline 55.3g, purity 99.7% (HPLC), yield 98.4%.
[0036] The mother liquor was cooled to 0 degree by suctio...
Embodiment 3
[0038] A preparation method of 2,6-dibromo-4-(trifluoromethoxy)aniline, comprising the following steps:
[0039] Put 350g of the mother liquor in which potassium sulfate crystals were filtered out in Example 2 into a 1000mL three-necked flask, start stirring and drop in 17.5 (0.175mol) sulfuric acid, then add 43.5g of potassium bromide (0.365mol) and 30g (99%, 0.168mol) 4-Trifluoromethoxyaniline, adjust the temperature above 30°C, start to add 38g (0.391mol) of 35% hydrogen peroxide dropwise, the temperature rises during the dropwise addition, when the temperature rises to 50°C, control the dropwise temperature by controlling the cooling liquid Keep the temperature between 50 and 55°C, keep the temperature for dripping, keep warm for 3.5 hours after dripping, cool down to about 30°C, filter, and dry the filter cake to obtain white crystal 2,6-dibromo-4-trifluoromethoxy Aniline 55.4g, purity 99.2% (HPLC), yield 98.1%.
[0040] The mother liquor was cooled to 0 degrees by sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com