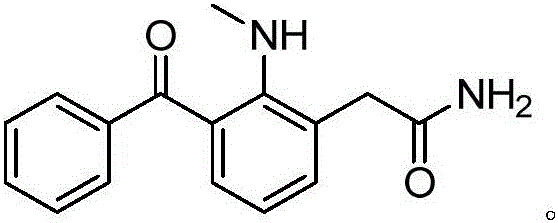
2-(3-benzyl-2-(dimethylamino) phenyl)acetamide, synthesis method and application thereof
A technology of dimethylamino and acetamide, applied in the field of the new compound 2‐(3‐benzyl‐2‐(2, can solve the problems affecting drug efficacy, side effects, etc., achieve high purity, simple operation, and ensure safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 2‐(3‐Benzyl‐2‐(dimethylamino)phenyl)acetamide
[0029] Add 5g (19.66mmol) of the compound shown in Formula a into a 250ml reaction flask, add 100ml of dichloromethane, stir to dissolve, add 4.19g (29.49mmol) methyl iodide dropwise at 0°C, after the drop is complete, heat to reflux for 3h. TLC detected that the reaction was complete, and the organic phase was concentrated under reduced pressure and recrystallized to obtain 3.1 g of a yellow solid, with a yield of 58.7%. 98.2% purity.
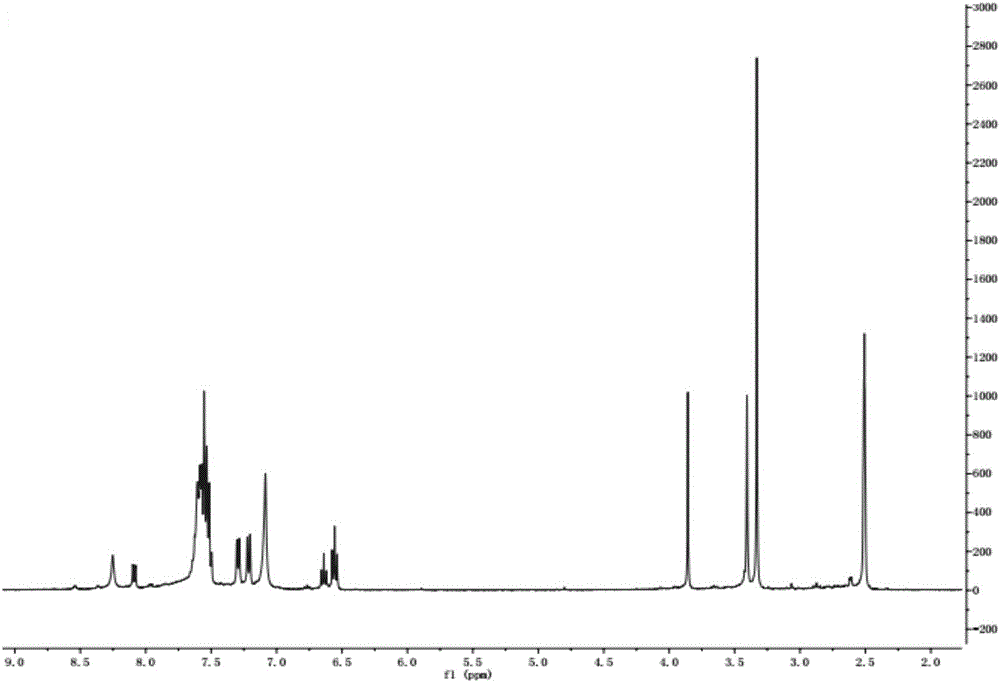
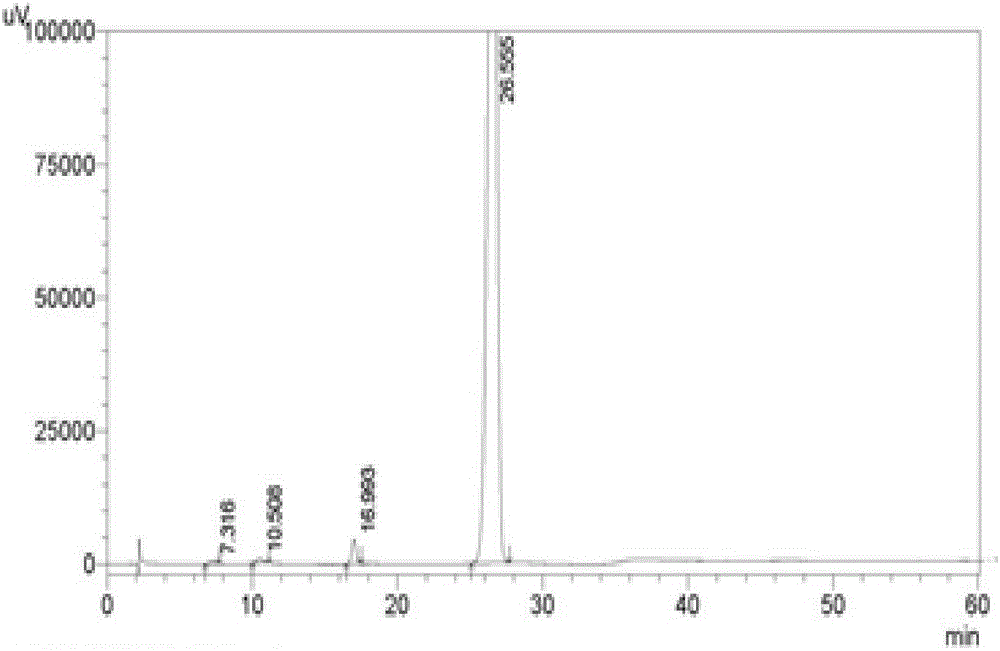
[0030] The liquid phase diagram of 2‐(3‐benzyl‐2‐(dimethylamino)phenyl)acetamide is shown in figure 1 As shown, it can be seen from the figure that the purity of 2-(3-benzyl-2-(dimethylamino)phenyl)acetamide is 98.2%; the prepared 2-(3-benzyl-2 ‐(Dimethylamino)phenyl)acetamide has a hydrogen spectrum as figure 2 shown.
Embodiment 2
[0031] Embodiment 2: the preparation of 2-(3-benzyl-2-(dimethylamino) phenyl) acetamide
[0032] Add 5g (19.66mmol) of the compound shown in formula a into a 250ml reaction flask, add 100ml tetrahydrofuran, stir to dissolve, add 3.22g (33.43mmol) dimethyl sulfate dropwise at room temperature, after the drop is completed, heat up to reflux for 6h, TLC After the reaction was completed, suction filtration was performed, and the filtrate was distilled under reduced pressure to obtain 4.9 g of solids, which were recrystallized to obtain 2.9 g of solids. The yield was 56.7%. The obtained compound is the same as in Example 1.
Embodiment 3
[0033] Embodiment 3: the preparation of 2-(3-benzyl-2-(dimethylamino) phenyl) acetamide
[0034] Add 5g (19.66mmol) of the compound shown in formula a into a 250ml reaction bottle, add 100ml ethyl acetate, stir to dissolve, add 3.79g (39.32mmol) dimethyl sulfate dropwise at room temperature, after the drop is completed, heat up to reflux for 2h , the TLC reaction was completed, suction filtered, and the filtrate was distilled under reduced pressure to obtain 4.9 g of solids, and recrystallized to obtain 3.0 g of solids. The yield was 57.7%. The obtained compound is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com