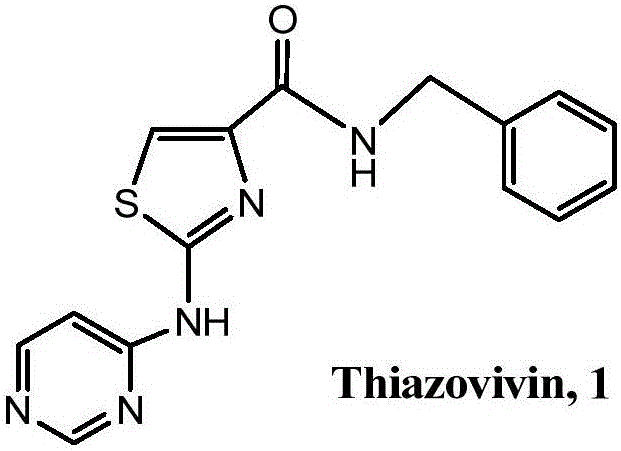
Ionic liquid and method for synthesizing Thiazovivin by same
An ionic liquid and compound technology, applied in the direction of organic chemistry, can solve the problems of complex operation, high cost, difficult monitoring, etc., and achieve the effect of simple operation, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1, ionic liquid of the present invention and preparation method thereof
[0074] The present embodiment provides a kind of ionic liquid for synthesizing Thiazovivin, it is characterized in that, described ionic liquid has the structure shown in following general formula 1:
[0075]
[0076] where R is selected from
[0077] x is selected from Cl, Br, I, BF 4 、PF 6 , ClO 4 、H 2 PO 4 , TS, ClO 4 , Lac, OAc, DCA, HSO 4 , NO 3 、HSO 4 , TFMS, NTF 2 , TSA, FSI;
[0078] n is any natural number in 0-10.
[0079] In the above general formula, the carbon chain can be shortened or lengthened, and the benzene ring part It is necessary, because this structure can selectively remove the ionic liquid by TFA when removing the ionic liquid, without affecting another benzyl group connected to the N atom; theoretically, This part of the structure can be in any form, the imidazole-type ionic liquid provided in the preferred embodiment of the present inventio...
Embodiment 2
[0088] Embodiment 2, the method for synthesizing Thiazovivin according to the present invention
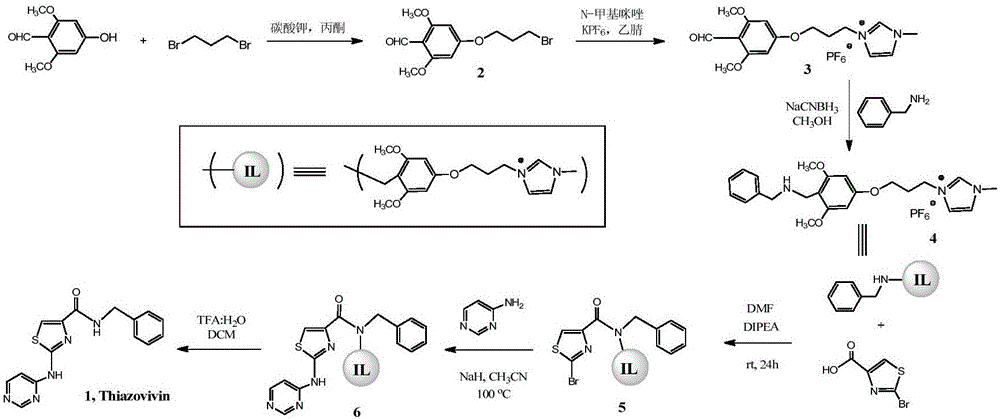
[0089] The present embodiment provides a method for synthesizing Thiazovivin, comprising the following steps:
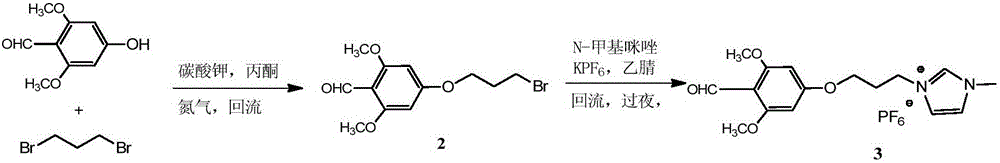
[0090] (1) prepare ionic liquid;
[0091] (2) connecting the ionic liquid with benzylamine to obtain compound 4;
[0092]
[0093] (3) Acylation reaction of the above compound 4 with 2-bromothiazole-4-carboxylic acid to obtain compound 5;
[0094]
[0095] (4) Amination of the above compound 5 with 4-aminopyrimidine to obtain compound 6;
[0096]
[0097] (5) The Thiazovivin can be obtained by removing the ionic liquid in the above compound 6;
Embodiment 3
[0098] Embodiment 3, using the method of the present invention to synthesize Thiazovivin (1)
[0099] Based on the ionic liquid provided in Example 2, this embodiment provides specific steps for synthesizing Thiazovivin by the method of the present invention, such as figure 2 shown, including the following steps:
[0100] Under nitrogen protection, using the ionic liquid (4.50 g, 10.0 mmol) provided in Example 2, benzylamine (2.2 ml, 20.0 mmol), sodium cyanoborohydride (NaCNBH 3 , 2.48 g, 40.0 mmol) was dissolved in dry methanol (100 mL). The reaction solution was stirred at room temperature for 12 hours. Thin layer chromatography showed that the reaction was complete. The solvent was evaporated to dryness under reduced pressure, then dissolved in dichloromethane (200 ml), and washed with 1M HCl, saturated NaHCO 3 and saturated NaCl extraction, organic phase with anhydrous NaCl 2 SO 4 After drying, filtering, and evaporating the solvent, the resulting mixture was purified...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com