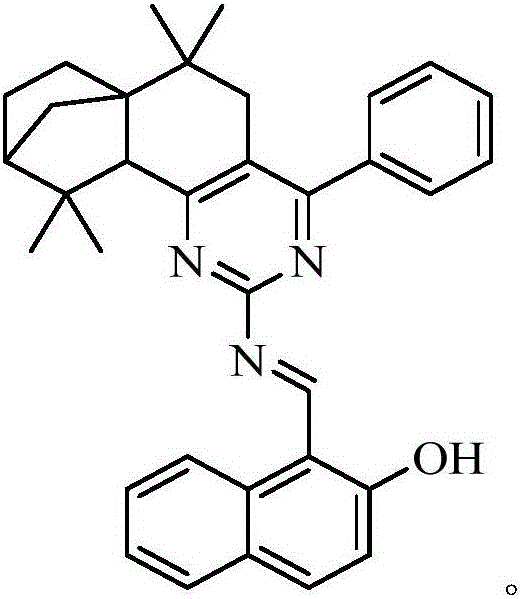
Iso-longitolanone based hexahydroquinazoline-2-amine Schiff base Zn<2+> fluorescent probe as well as preparation method and application thereof
A kind of technology of felanone-based hexahydroquinazoline and amine Schiff base, which is applied in the field of fine organic synthesis and can solve the problem of not synthesizing hexahydroquinazoline-2-amine Schiff base compound and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic method of isolongifanonyl hexahydroquinazolin-2-amine Schiff base compound, the synthetic process is:
[0033]
[0034] Specific steps are as follows:
[0035]1) Preparation of 7-benzylidene isolongifanone:
[0036] Add 2.2g of isolongifolanone, 1.06g of benzaldehyde, 0.51g of sodium ethoxide and 30mL of ethanol into a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser in sequence, and heat to reflux at 80-90°C for reaction. After about 4 hours of reaction, the conversion rate of isolonganone reaches over 95% (GC tracking detection). The reactant was extracted 3 times with 20 mL of ethyl acetate, the organic phases were combined, and washed several times with saturated brine until neutral; the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to recover the solvent to obtain 7-benzylidene iso The crude longifolanone was recrystallized from ethanol-ethyl acetate to obtain colorless and trans...
Embodiment 2
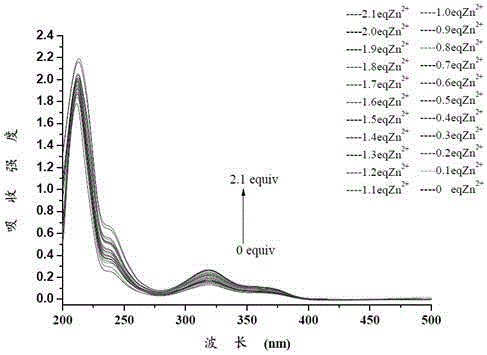
[0043] A certain amount of 1-((((6,6,10,10-tetramethyl-4-phenyl-5,7,8,9,10,10α-hexahydro-6H-6α,9-methanomethene Benzo[h]-2-quinolyl)imino)methyl)naphthalene-2-ol was dissolved in HEPES buffer solution (1×10 -5 M), the same Zn 2+ Ions are dissolved in HEPES buffer solution to make a concentration of 0~2.1×10 -5 M's solution. Different concentrations of Zn were measured 2+ Ion pair 1-(((6,6,10,10-tetramethyl-4-phenyl-5,7,8,9,10,10α-hexahydro-6H-6α,9-methanobenzene And [h]-2-quinolinyl)imino)methyl)naphthalene-2-ol's ultraviolet absorption spectrum, such as figure 1 shown. The results show that the ultraviolet absorption of the compound at around 320nm is significantly enhanced, indicating that the compound can interact with Zn 2+ The ions are complexed.
Embodiment 3
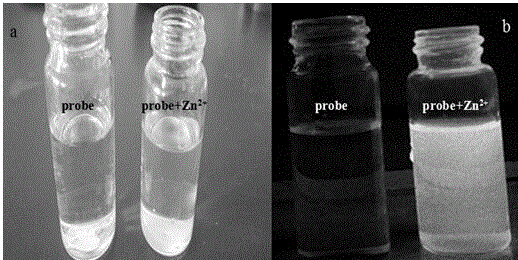
[0045] A certain amount of 1-((((6,6,10,10-tetramethyl-4-phenyl-5,7,8,9,10,10α-hexahydro-6H-6α,9-methanomethene Benzo[h]-2-quinolyl)imino)methyl)naphthalene-2-ol was dissolved in HEPES buffer solution (1×10 -5 M), its solution is colorless and transparent, along with Zn 2+ Ions (1×10 -5 M) adding, the solution becomes bright yellow rapidly, observes under 365nm ultraviolet light, as figure 2 Shown, a: compound added Zn under sunlight 2+ Photos of before and after ions; b: compound added Zn under 365nm UV light 2 + Ion before and after photos, adding Zn 2+ The solution of ions emits a strong green fluorescence, and by adding an equimolar amount of K + , Na + , Cu 2+ , Hg 2+ , Pb 2+ , Mg 2+ , Fe 3+ , Fe 2+ , Ni 2+ , Cd 2+ ,Co 2+ , Mn 2+ and other metal ions, it will not lead to the enhancement of the fluorescence of the compound, indicating that the compound can be used as an effective recognition Zn 2+ Ionic fluorescent probes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com