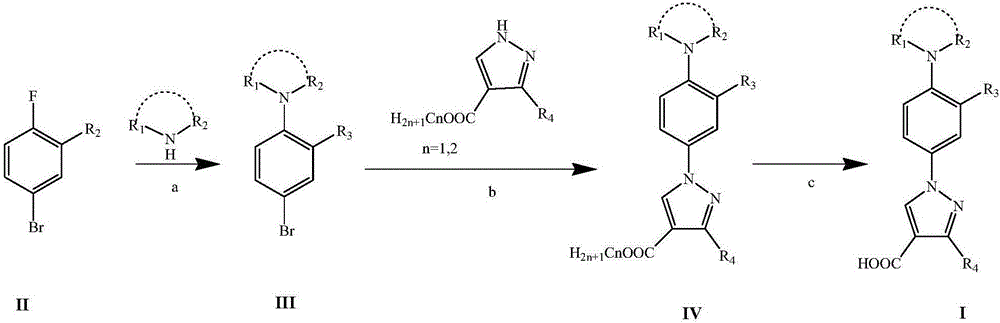
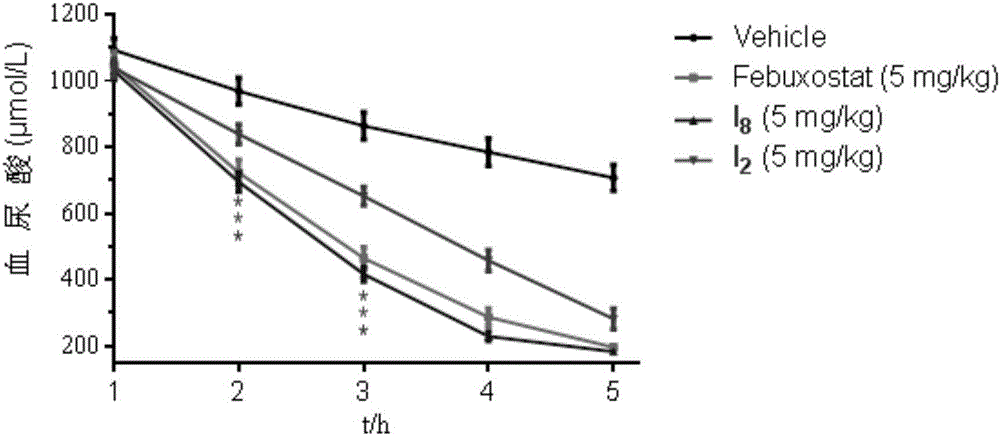
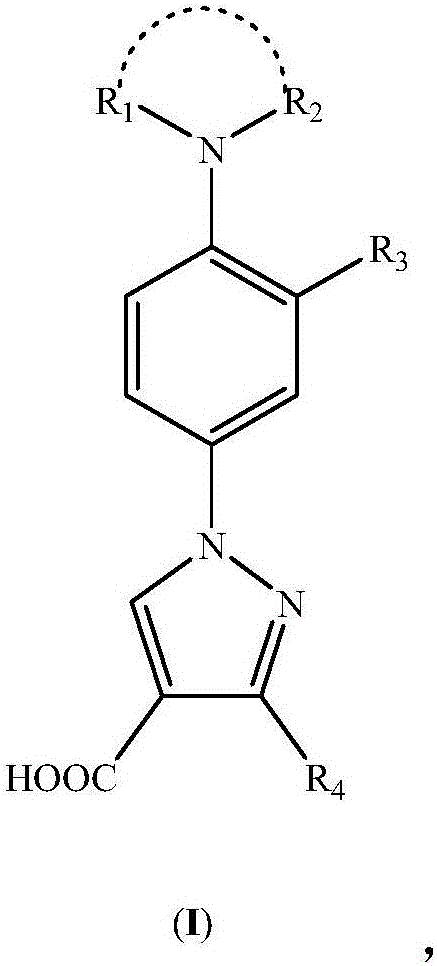
Nitrogen-substituted phenyl pyrazole xanthine oxidoreductase inhibitor and preparation and application thereof
A technology of phenylpyrazoles and reductase inhibitors, which is applied in the field of nitrogen-substituted phenylpyrazole xanthine oxidoreductase inhibitors and their preparation and application, and can solve urate crystal deposition, kidney damage, etc. problem, to achieve the effect of excellent inhibitory effect and excellent inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1-(3'-cyano-4'-piperidine-phenyl)-3-methyl-pyrazole-4-carboxylic acid (I 1 )Synthesis
[0058] (1) 5-bromo-2-fluoro-1-cyanobenzene (II 1 , 2.64g, 13mmol) was dissolved in DMSO (20mL), adding K 2 CO 3 (5.4g, 39mmol), piperidine (3.3g, 39mmol), reacted at 100°C for 6h, followed by TLC to complete the reaction. The reaction solution was cooled to room temperature, diluted with 100 mL of water, extracted with ethyl acetate (100 mL×3), washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to remove the solvent under reduced pressure to obtain a yellow liquid 5-bromo-2-piperidine- Benzonitrile (III 1 )3.3g, the yield is 95.7%.
[0059] (2)N 2 Under protection, 3-methyl-1H-pyrazole-4-carboxylic acid methyl ester (0.14g, 1.0mmol), CuI (19.1mg, 0.1mmol), K 2 CO 3 (0.29g, 2.1mmol), III 1 (0.318g, 1.2mmol), (E)-N,N'-dimethyl-1,2-cyclohexyldiamine (28.5mg, 0.2mmol) and DMF (3mL) were added in a 25mL two-necked flask, 110 React at ℃ for 24 h, ...
Embodiment 2
[0063] 1-(3'-cyano-4'-piperidine-phenyl)-pyrazole-4-carboxylic acid (I 2 )Synthesis
[0064] (1) With embodiment 1.
[0065] (2)N 2 Under protection, ethyl 1H-pyrazole-4-carboxylate (0.22g, 1.6mmol), CuI (30mg, 0.16mmol), K 2 CO 3 (0.45g, 3.3mmol), III 1 (0.5g, 1.9mmol), (E)-N,N'-dimethyl-1,2-cyclohexyldiamine (45mg, 0.3mmol) and DMF (3mL) were added to a 25mL two-neck flask, 110°C React for 24 hours, cool to room temperature, add 10 mL of water to dilute, extract with ethyl acetate (15 mL×3), wash with saturated brine (10 mL), dry over anhydrous magnesium sulfate, evaporate the solvent under reduced pressure, and purify on a silica gel column (V 乙酸乙酯 :V 石油醚 =4:1), white solid 1-(3'-cyano-4'-piperidine-phenyl)-pyrazole-4-carboxylic acid ethyl ester (IV 2 )0.28g, yield 55.0%.
[0066] (3)IV 2 (0.28g, 0.86mmol) was dissolved in a mixed solution of THF (24mL) and ethanol (24mL), added 4mL of 1M NaOH aqueous solution, refluxed for 1h, cooled to room temperature, added 1M ...
Embodiment 3
[0069] 1-(3'-nitro-4'-piperidine-phenyl)-3-methyl-pyrazole-4-carboxylic acid (I 3 )Synthesis
[0070] (1) 5-bromo-2-fluoro-1-nitrobenzene (II 2 , 3.3g, 15mmol) was dissolved in DMSO (20mL), adding K 2 CO 3 (6.2g, 45mmol), piperidine (3.8g, 45mmol), reacted at 100°C for 4h, followed by TLC to complete the reaction. The reaction solution was cooled to room temperature, diluted with 100 mL of water, extracted with ethyl acetate (100 mL×3), washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to remove the solvent under reduced pressure to obtain 5-bromo-2-piperidine as an orange liquid -1-nitrobenzene (III 3 )4.2g, the yield is 98.2%.
[0071] (2)N 2 Under protection, 3-methyl-1H-pyrazole-4-carboxylic acid methyl ester (0.14g, 1.0mmol), CuI (19.1mg, 0.1mmol), K 2 CO 3 (0.29g, 2.1mmol), III 3 (0.34g, 1.2mmol), (E)-N,N'-dimethyl-1,2-cyclohexyldiamine (28.5mg, 0.2mmol) and DMF (3mL) were added in a 25mL two-necked flask, 110 React at ℃ for 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com