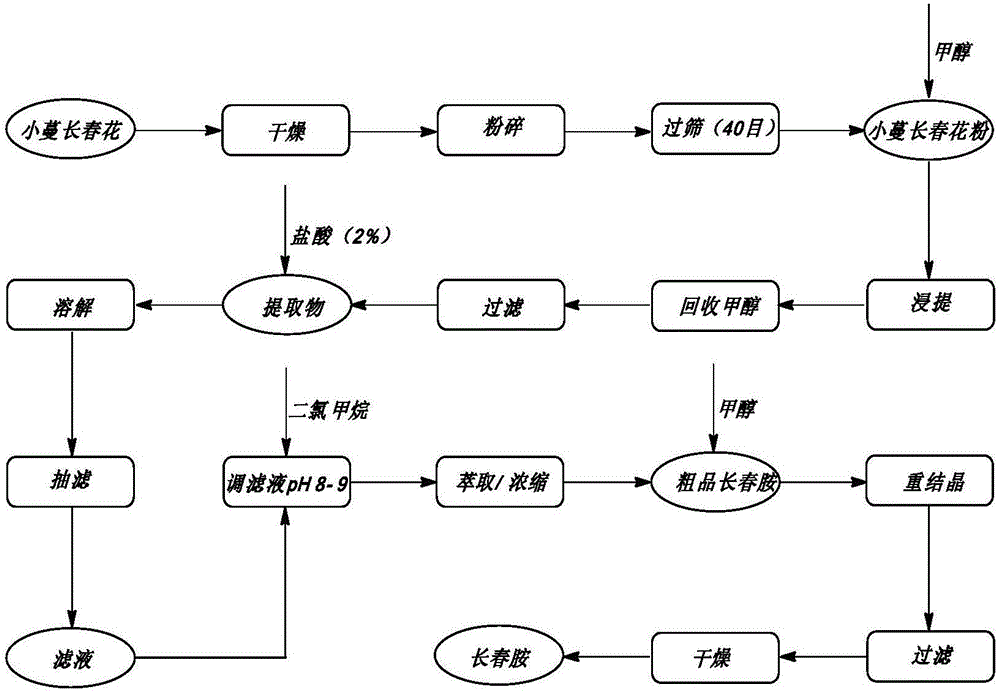
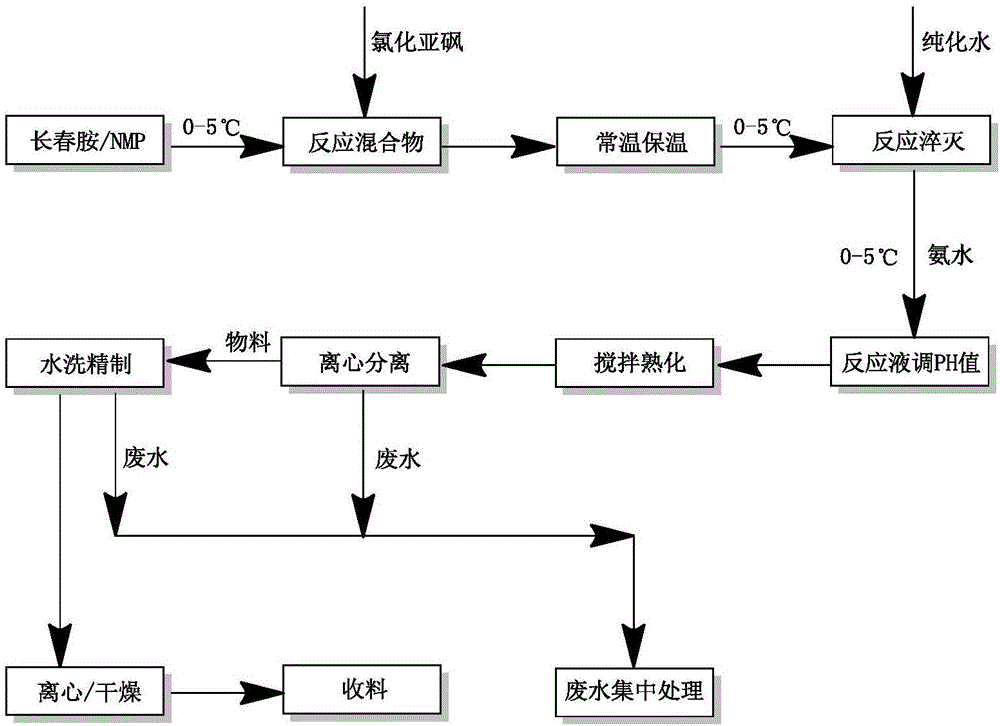
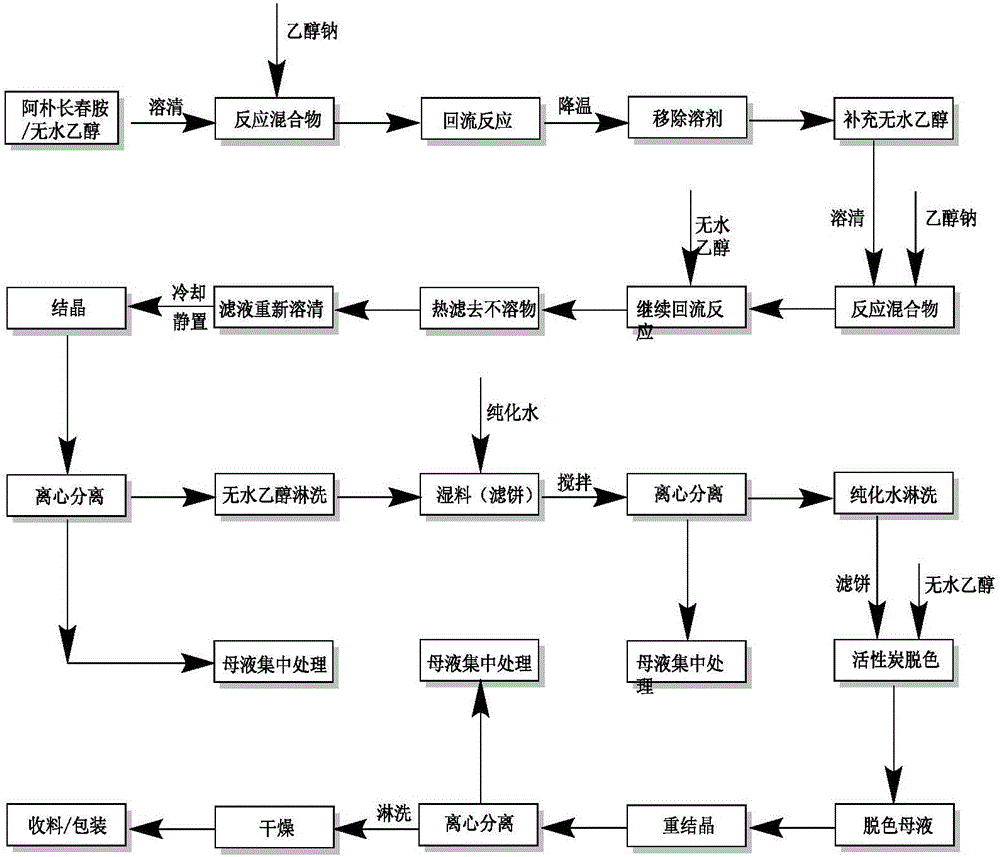
Method for preparing vinpocetine
A technology of vinpocetine and vincamine, which is applied in the field of compound preparation, can solve the problems of three wastes treatment and adverse effects of solvent residues, unsuitable for large-scale industrial production, and short routes, etc., and achieves good reproducible results, easy handling, and less residues Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] This implementation takes vincamine as raw material, first prepares apovincamine, and then prepares vinpocetine with apovincamine as raw material.
[0099] The method that present embodiment prepares Vinpocetine comprises the steps:
[0100] 1) preparation of apovincamine
[0101] Put 15mL of NMP and 5g of vincamine into a 50mL dry and clean single-necked flask, stir for 5-10 minutes to obtain a uniform mixture, put an external ice-water bath to cool down to 0-5°C, add 1.62mL of thionyl chloride dropwise, and control the internal temperature. When the temperature exceeds 20°C, the dropwise addition is completed in half an hour. The ice bath was removed, and the temperature was raised to normal temperature (RT, 20-25° C.) in about 30 minutes, and the reaction was stirred for 30 minutes. The reaction was monitored by HPLC, and the reaction was terminated when the remaining amount of raw material vincamine was less than 1%. Then lower the temperature to 0-5°C, add 10mL ...
Embodiment 2
[0108] The purity of vincamine in this embodiment is not less than 99.3%.
[0109] This implementation uses vincamine as a raw material, firstly prepares apovincamine, and then prepares vinpocetine by using apovincamine as a raw material.
[0110] The method for preparing vinpocetine in the present embodiment comprises the following steps:
[0111] 1) Preparation of apovincamine
[0112] Put 690 mL of NMP and 230 g of vincamine in a 2000 mL dry and clean single-neck flask, stir for 5-10 minutes to obtain a uniform mixture, cool down to 0-5 ℃ in an external ice-water bath, add 74.8 mL of thionyl chloride dropwise, and control the internal temperature Above 20°C, the dripping is completed in about 1 hour. Remove the ice bath, stir and heat up to normal temperature (RT, 20-25 ℃) for about half an hour, monitor the residual amount of vincamine in the reaction with HPLC, end the reaction when the residual is less than 1%, then cool down to 0-5 ℃, add dropwise Purified water 460m...
Embodiment 3
[0120] The purity of vincamine in this embodiment is not less than 99.3%.
[0121] This implementation uses vincamine as a raw material, firstly prepares apovincamine, and then prepares vinpocetine by using apovincamine as a raw material.
[0122] The method for preparing vinpocetine in the present embodiment comprises the following steps:
[0123] 1. Preparation of apovincamine
[0124] Put 690 mL of NMP and 230 g of vincamine in a 2000 mL dry and clean single-neck flask, stir for 5-10 minutes to obtain a uniform mixture, cool down to 0-5 ℃ in an external ice-water bath, add 74.8 mL of thionyl chloride dropwise, and control the internal temperature Above 20°C, the dripping is completed in about 1 hour. Remove the ice bath, stir and heat up to normal temperature (RT, 20-25 ℃) for about half an hour, monitor the residual amount of vincamine in the reaction with HPLC, end the reaction when the residual is less than 1%, then cool down to 0-5 ℃, add dropwise Purified water 460m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com