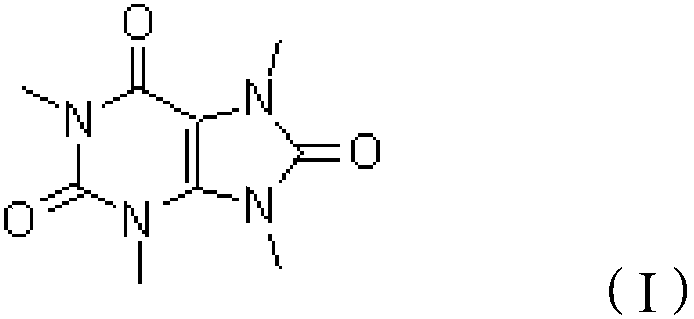The preparation method of 1,3,7,9-tetramethyluric acid
A technology of tetramethyluric acid and ethyl acetate, applied in the direction of organic chemistry, can solve the problems of low sample yield, small preparation amount, large pollution, etc., and achieve the effect of retaining biological activity, large preparation amount, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of 1,3,7,9-tetramethyluric acid in this embodiment comprises the following steps:
[0033] S1, get bitter tea extract 11.9g as the sample to be separated; Preparation volume ratio is the solvent system of ethyl acetate, n-butanol and water of 1:1.5:3, and described solvent system comprises upper stratum and lower strata, in described upper strata Add the triethylamine of 10mmol / L as stationary phase, add the hydrochloric acid of 10mmol / L in the lower layer as mobile phase, get 120mL described mobile phase and dissolve described sample to be separated, obtain the solution to be separated;
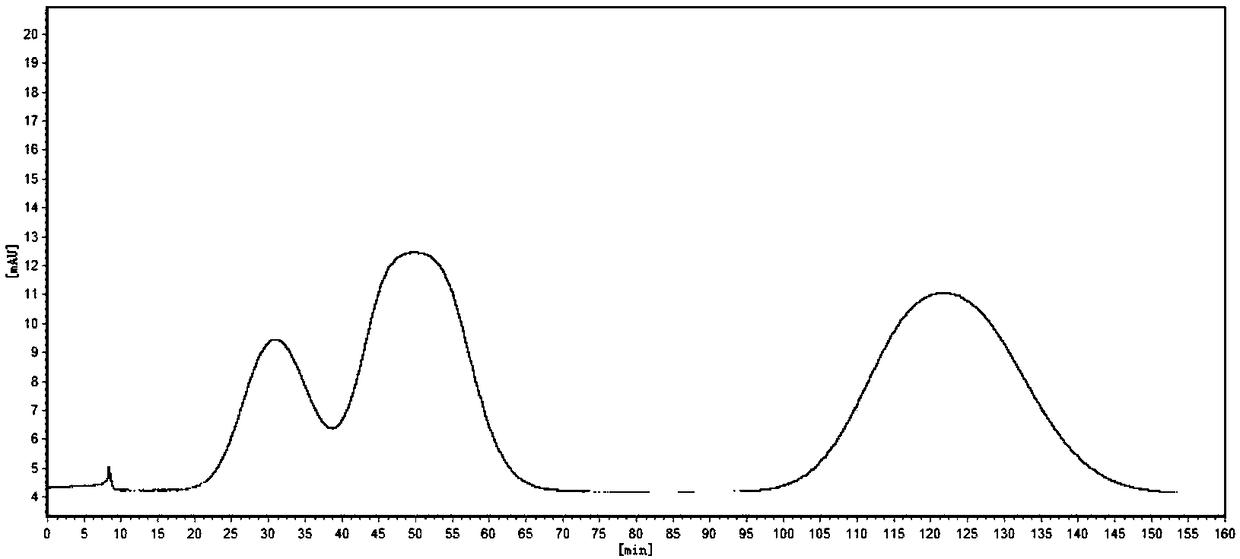
[0034] S2. Pump the stationary phase and mobile phase into the chromatographic column of high-speed countercurrent chromatography, the head end goes in, and the tail end goes out; The countercurrent chromatogram obtained by the recorder determines whether 1,3,7,9-tetramethyluric acid (appearing figure 2 The chromatogram shown shows that the fractions contain 1,3...
Embodiment 2
[0036] In this example, a method for preparing 1,3,7,9-tetramethyluric acid, the steps are similar to Example 1, the difference is that the solvent system in step S1 is ethyl acetate with a volume ratio of 1.5:0.5:1, n-butanol and water.
Embodiment 3
[0038] In this example, a method for preparing 1,3,7,9-tetramethyluric acid, the steps are similar to Example 1, the difference is that the solvent system in step S1 is ethyl acetate with a volume ratio of 0.5:2:6, n-butanol and water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com