Preparation method of SOD active drug carrier wrapped with novel microsphere biological material
A technology of active drugs and new materials, applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as low recovery rate, degradation, and frequent administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
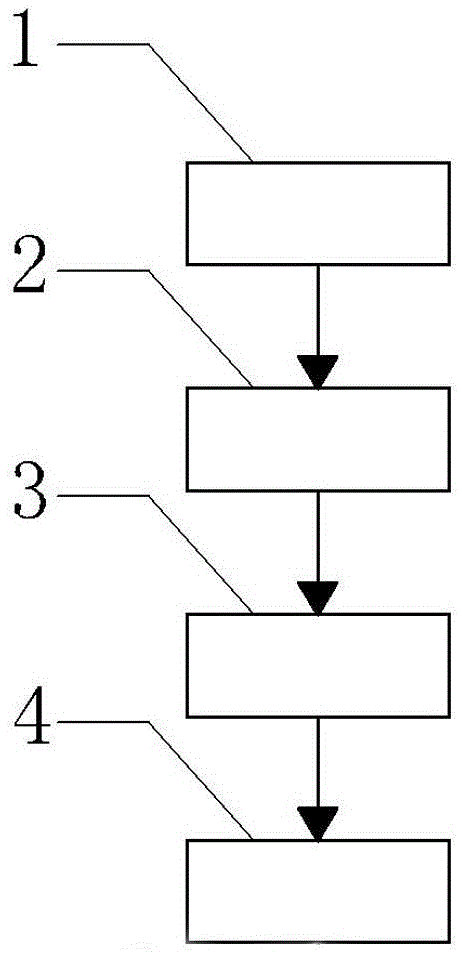
[0022] It can be seen from the contents of the invention that a new microsphere biological material wraps SOD drug carrier, which consists of the preparation of blank chitosan microspheres 1, the preparation of SOD chitosan microspheres 2, the drug loading of SOD chitosan microspheres and their Determination of encapsulation efficiency 3, SOD chitosan microsphere drug release test in vitro 4 four steps to complete its preparation method:
[0023] Preparation of blank chitosan microspheres 1: Dissolve 100 g of chitosan in 1000 mL of 2% glacial acetic acid solution, stir magnetically, add Tween-80 1.0 mL, magnetically stir and sonicate, add 30% Na 2 SO 4Solution, until the above solution is turbid, measure the turbidity at 500 min by UV spectrophotometer to determine the formation of microspheres, continue stirring and ultrasonic treatment for 1 h after microspheres are formed, centrifuge, take 10000~12000 r / min, centrifuge After 15 min, the resulting precipitate was resuspende...
Embodiment 2
[0031] Anti-cancer SOD microsphere drug experiment
[0032] (1) Suspend 100 mg of freeze-dried chitosan microspheres in 250 mL of acetate buffer (pH = 6.2), add 5×10 3 U / mLSOD 1.0mL, magnetically stirred at 4°C, centrifuged at 10000~12000r / min to precipitate, the precipitate (microsphere) was washed 3 times with deionized water, dried in vacuum, and stored at 4°C; the precipitate was washed again, purified, and freeze-dried; Collect and combine the centrifugate and washing liquid to obtain drug-loaded microspheres, and the dispersion is called "SOD microsphere combined liquid"; the SOD content in chitosan microspheres is 5KU=10mg or 5.31×10 4 U / g, the encapsulation efficiency was 97.3%; 50000U / g-SOD microspheres were obtained by freeze-drying, and the senses were light blue-green or white dense spheres.
[0033] (2) Natural cumulative drug release rate of magnetic anti-cancer microspheres: Accurately weigh 50 mg of anti-cancer SOD microspheres, 4 parts in total; wash the oil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com