Divalent manganese ion activated fluorescent powder, a preparing method thereof and applications of the fluorescent powder
A technology of divalent manganese ions and phosphors, applied in the field of phosphors, can solve the problems of easy high-temperature oxidation, high cost, complicated reaction operations and the like of fluorescent materials, and achieve the effects of easy promotion, low cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Take by weighing 0.005mol purity and be 99.9% sodium carbonate, (0.01-x) mol purity be the zinc oxide of 99.9%, 0.03mol purity be the ammonium dihydrogen phosphate of 99.5%, (x) mol purity be the manganese dioxide of 99.99% , where x is 0.00001~0.0017.
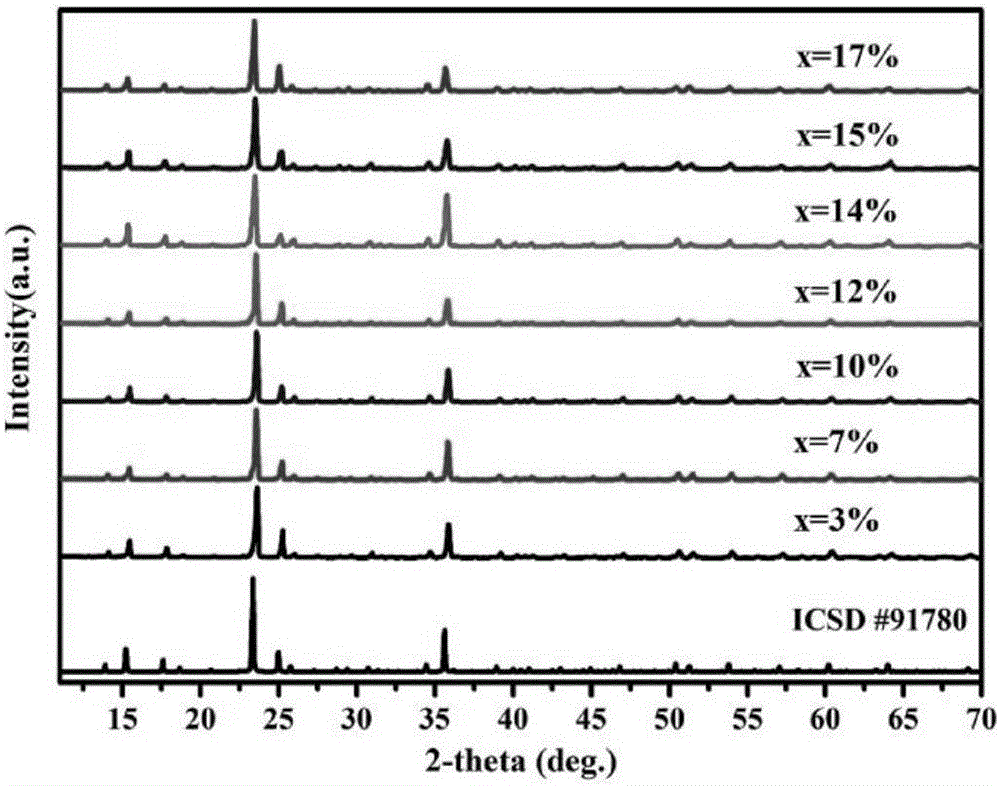
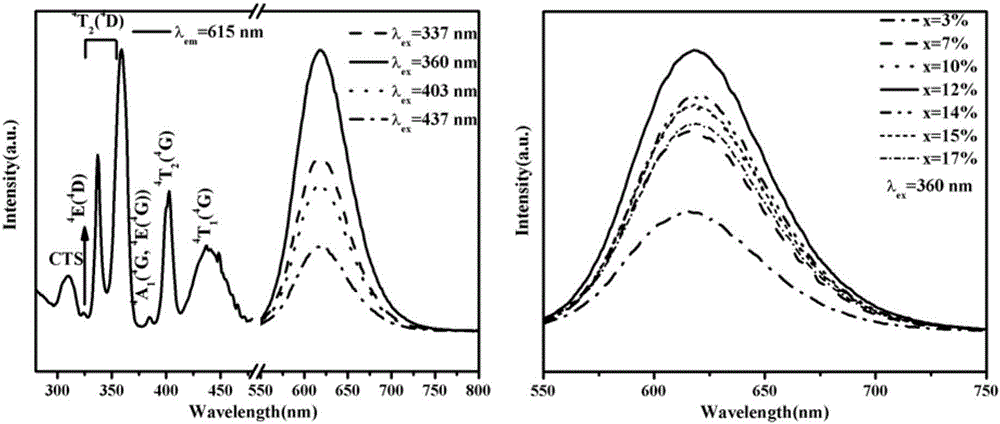
[0046] Put the above raw materials into a mortar and grind them evenly, put them into a corundum crucible and a platinum crucible respectively, put the crucible in a muffle furnace, raise it from room temperature to 500°C at a rate of 4°C / min, and heat it at 500°C Keep the temperature constant for 24 hours, then cool down to room temperature naturally. Take out the sample and place it in an agate mortar and grind it fully again, put it back into the crucible, put it in a muffle furnace, heat it to 800°C, keep the temperature constant for 48 hours, take it out and grind it again evenly after cooling down naturally. Both crucibles can get the white target product NaZn 1-x (PO 3 ) 3 :xMn 2+ (x=0.001~0.17) powder.
[...
Embodiment 2
[0049] Take by weighing 0.005mol purity and be 99.9% sodium carbonate, (0.01-x) mol purity be the magnesium oxide of 99.9%, 0.03mol purity be the ammonium dihydrogen phosphate of 99.5%, (x) mol purity be the manganese dioxide of 99.99% , where x is 0.00001~0.0017.
[0050] Put the above raw materials into a mortar and grind them evenly, then put them into a corundum crucible and a platinum crucible respectively, place the crucible in a muffle furnace, raise it from room temperature to 500°C at a rate of 4°C / min, and keep the temperature at 500°C 24 hours, then cool down to room temperature naturally. Take out the sample and place it in an agate mortar and grind it fully again, put it back into the crucible, put it in a muffle furnace, heat it to 800°C, keep the temperature constant for 48 hours, take it out and grind it again evenly after cooling down naturally. Both crucibles can get the white target product NaMg 1-x (PO 3 ) 3 :xMn 2+ (x=0.001~0.17) powder.
[0051] The...
Embodiment 3
[0053] Take by weighing 0.01mol purity be the lithium carbonate of 99.9%, (0.01-x) mol purity is the zinc oxide of 99.9%, 0.01mol purity is the ammonium dihydrogen phosphate of 99.5%, (x) mol purity is the manganese dioxide of 99.99% , where x is 0.00001~0.0016.
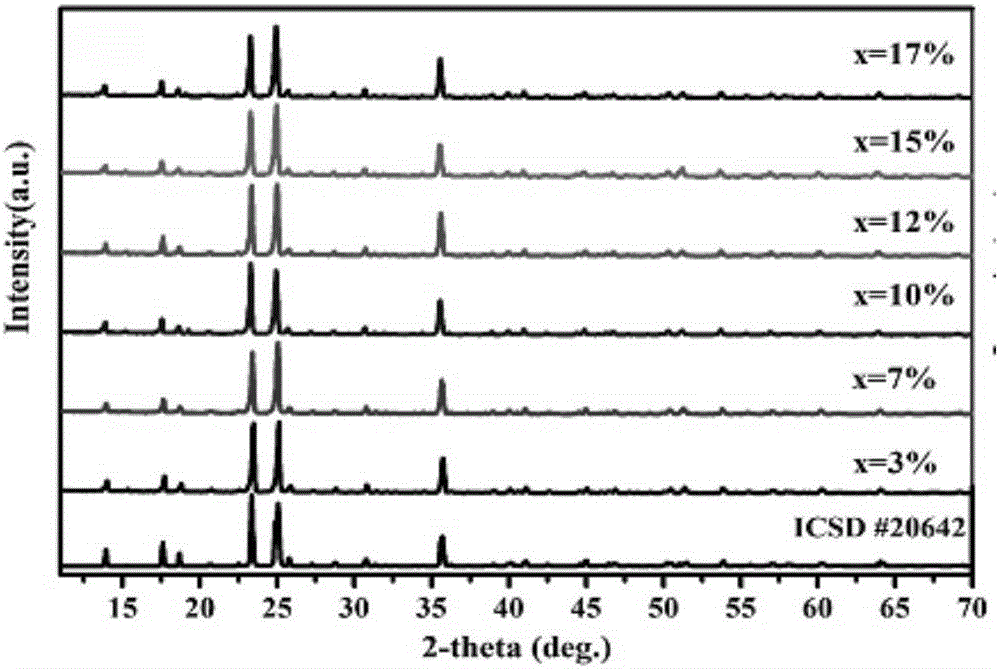
[0054] Put the above raw materials into a mortar and grind them evenly, then put them into a corundum crucible and a platinum crucible respectively, place the crucible in a muffle furnace, raise it from room temperature to 500°C at a rate of 4°C / min, and keep the temperature at 500°C 24 hours, then cool down to room temperature naturally. Take out the sample and place it in an agate mortar and grind it fully again, put it back into the crucible, put it in a muffle furnace, heat it to 800°C, keep the temperature constant for 48 hours, take it out and grind it again evenly after cooling down naturally. Both crucibles can get the white target product LiZn 1-x PO 4 :xMn 2+ (x=0.001~0.16) powder.
[0055] Then, to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com