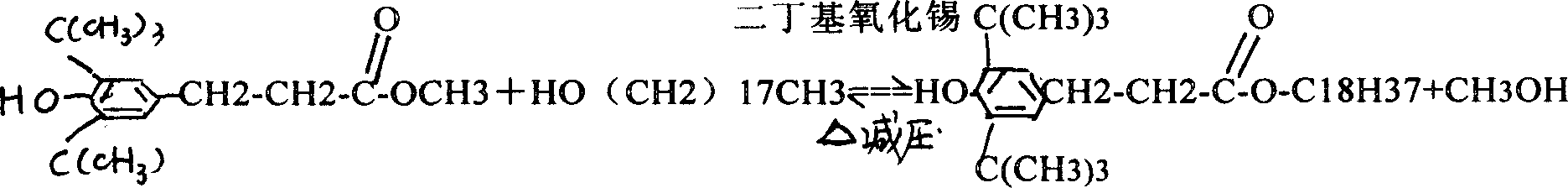
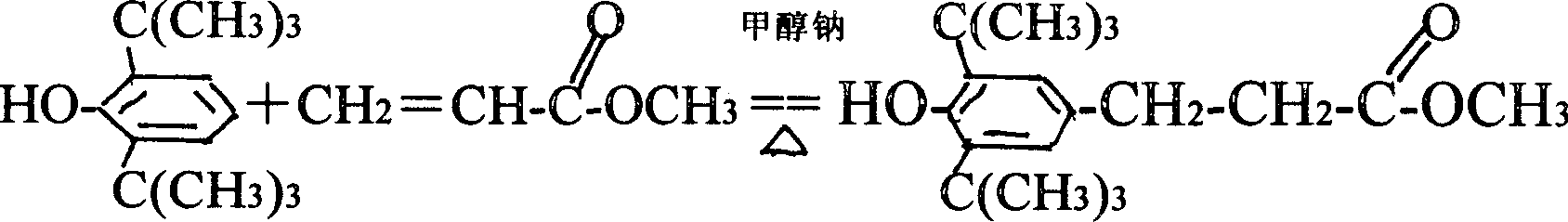Two-step industrial synthesis method of beta-(3,5-di tertiary butyl-4-hydroxyl phenyl )octadecyl propionate
A technology of stearyl propionate and hydroxyphenyl, which is applied in the synthesis field of β-stearyl propionate, can solve the problems of deepening the color of the reaction solution, decreasing the quality of the product, limiting the efficiency, etc. Quality improvement, the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]558kg (55°C) of preheated 2.6 di-tert-butylphenol was sucked into the addition reaction kettle by negative pressure, and at the same time, the stirring was started, and nitrogen was filled; 16.74Kg of catalyst sodium methoxide was added, and dripped into the addition reaction kettle Add 280Kg of methyl acrylate, and the time for the dropwise addition is 30 minutes. During the dropwise addition, the reaction temperature is kept between 90-110° C. After the dropwise addition, the temperature of the addition reactor was kept at 110-124° C., and the reaction ended after 4 hours. Add 27Kg of acetic acid to the addition reactor for neutralization for 15 minutes, add 400Kg of aqueous 14% methanol solution to dissolve, and transfer it to a crystallization tank at 65°C for crystallization and separation to obtain 705Kg of 3.5 methyl esters.
[0042] Put 230Kg of the above 3.5 methyl esters, 200Kg of stearyl alcohol, and 3Kg of catalyst dibutyltin oxide into the transesterificatio...
Embodiment 2
[0046] 558kg (55°C) of preheated 2.6 di-tert-butylphenol was sucked into the addition reaction kettle by negative pressure, and at the same time, stirring was started, and nitrogen was filled; 14Kg of catalyst sodium methoxide was added, and added dropwise 255Kg of methyl acrylate was added dropwise for 25 minutes. During the dropwise addition, the reaction temperature was maintained at 90°C. After the dropwise addition, the temperature of the addition reactor was kept at 110° C., and the reaction ended after 4 hours. Add 17Kg of acetic acid to the addition reactor at 78°C for neutralization for 15 minutes until pH = 6.5, add 340Kg of 13.8% aqueous methanol solution to dissolve it, and transfer it to the crystallization kettle at 65°C for crystallization and separation to obtain 3.5 methyl ester 700Kg.
[0047] Put 210Kg of the above 3.5 methyl esters, 200Kg of stearyl alcohol, and 2Kg of catalyst dibutyltin oxide into the transesterification kettle successively. The tempera...
Embodiment 3
[0051] 558kg (55°C) of preheated 2.6 di-tert-butylphenol was sucked into the addition reaction kettle by negative pressure, and at the same time, stirring was started, and nitrogen was filled; 20Kg of catalyst sodium methoxide was added, and added dropwise 300Kg of methyl acrylate was added dropwise for 25 minutes. During the dropwise addition, the reaction temperature was maintained at 105°C. After the dropwise addition, the temperature of the addition reactor was kept at 124° C., and the reaction ended after 4.5 hours. At 80°C, add 30Kg of acetic acid to the addition reaction kettle for neutralization for 15 minutes to make the pH = 6.5; add 500Kg of 14% aqueous methanol solution to dissolve the reaction product and soluble salt, and transfer it to the crystallization kettle at 65°C Crystallization and separation were carried out to obtain 700Kg of 3.5 methyl esters.
[0052] Put 250Kg of the above 3.5 methyl esters, 200Kg of stearyl alcohol, and 4Kg of catalyst dibutyltin ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com