Method for preparing cyclopentanol by hydrating cyclopentene
A technology for hydration of cyclopentene and cyclopentanol is applied in the directions of hydrolysis preparation, preparation of organic compounds, preparation of carboxylate, etc., and can solve the problems of large circulation amount of raw material cyclopentene, complicated process, low reaction conversion rate and the like , to achieve the effect of high product yield, simple process flow and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
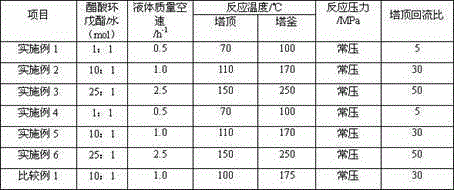
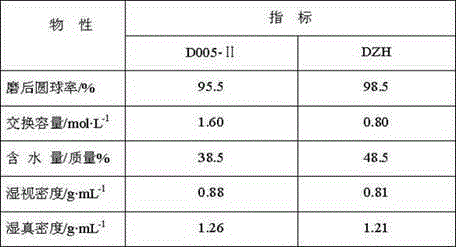
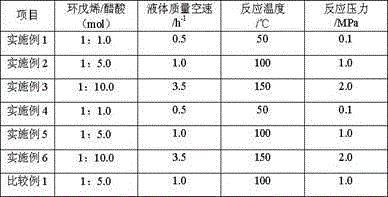
[0020] The commercially available D005-II sulfonic acid group cation exchange resin produced by Dandong Mingzhu Special Resin Co., Ltd. was soaked in toluene at 40°C for 28h, filtered and then soaked in methyl isobutyl ketone at 50°C for 40h. Addition reaction catalyst is obtained by washing and drying after filtering. The volume ratio of toluene or methyl isobutyl ketone to sulfonic acid cation exchange resin is 10:1. Take the above-mentioned addition reaction catalyst and put it into a Φ18mm×1200mm stainless steel reactor. The top and bottom of the reactor are respectively filled with quartz sand with a diameter of Φ0.5mm~1.2mm. After the reactor is installed, replace it with nitrogen three times, and The airtight test is qualified. Pass through cyclopentene and acetic acid to carry out addition reaction, the addition reaction conditions are shown in Table 2, and the addition reaction results are shown in Table 4. The above-mentioned addition reaction product and water ente...
Embodiment 2
[0022]Soak the commercially available DZH sulfonic acid-based cation exchange resin produced by Dandong Mingzhu Special Resin Co., Ltd. in toluene at 120°C for 10 hours, and then soak it in methyl isobutyl ketone at 80°C for 28 hours after filtration. After washing and drying, the addition reaction catalyst is obtained. The volume ratio of toluene or methyl isobutyl ketone to sulfonic acid-based cation exchange resin is 30:1. Take the above-mentioned addition reaction catalyst and put it into a Φ18mm×1200mm stainless steel reactor. The top and bottom of the reactor are respectively filled with quartz sand with a diameter of Φ0.5mm~1.2mm. After the reactor is installed, replace it with nitrogen three times, and The airtight test is qualified. Pass through cyclopentene and acetic acid to carry out addition reaction, the addition reaction conditions are shown in Table 2, and the addition reaction results are shown in Table 4. The above-mentioned addition reaction product and wat...
Embodiment 3
[0024] The commercially available D005-II sulfonic acid group cation exchange resin produced by Dandong Mingzhu Special Resin Co., Ltd. was soaked in toluene at 100°C for 15h, filtered and then soaked in methyl isobutyl ketone at 70°C for 35h. After filtering, washing and drying, the addition reaction catalyst is obtained. The volume ratio of toluene or methyl isobutyl ketone to sulfonic acid cation exchange resin is 20:1. Take the above-mentioned addition reaction catalyst and put it into a Φ18mm×1200mm stainless steel reactor. The top and bottom of the reactor are respectively filled with quartz sand with a diameter of Φ0.5mm~1.2mm. After the reactor is installed, replace it with nitrogen three times, and The airtight test is qualified. Pass through cyclopentene and acetic acid to carry out addition reaction, the addition reaction conditions are shown in Table 2, and the addition reaction results are shown in Table 4. The above-mentioned addition reaction product and water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com