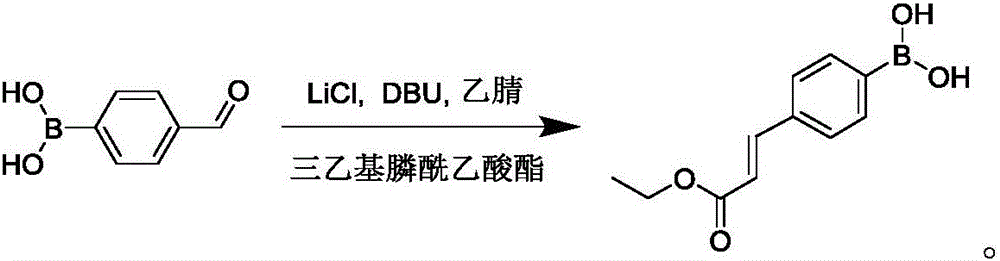
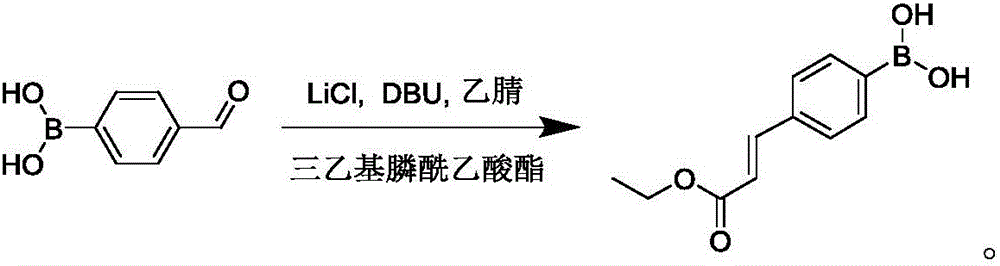
Preparation method of 4-(E3-ethoxyl-3-oxo-1-propylene-1-yl) borophenylic acid
A technology of phenylboronic acid and formylphenylboronic acid, which is applied in the field of pharmaceutical intermediates, can solve the problems of complex processing procedures, pollution, harsh conditions, etc., and achieve the effect of simple post-treatment process, high atom economy and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a 250mL three-neck flask, dissolve lithium chloride (4.2g, 100.0mmol) and DBU (15.2g, 100.0mmol) in acetonitrile (50mL), and add triethylphosphonoacetic acid at 0°C under N2 protection The ester (22.4g, 100.0mmol) was stirred for 10min, raised to room temperature and stirred for 30min, then added 4-formylphenylboronic acid (5.0g, 33.3mmol), and reacted at room temperature for 5h. After the reaction is complete, pour the reaction solution into water, extract with ethyl acetate (50mL×3), combine the organic layers, dry over anhydrous sodium sulfate, concentrate under reduced pressure, add concentrated hydrochloric acid (2mL) and a solid is precipitated, suction filtered, and the filter cake Slurry with a small amount of n-hexane (5 mL) and dry to obtain 5.8 g of 4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid with a yield of 78.8%.
[0018] 1 H NMR (400MHz, DMSO) δ7.82(d, J=8.2Hz, 2H), 7.67(d, J=7.4Hz, 2H), 7.62(s, 1H), 6.66(d, J=16.1Hz, 1H ), 4.20 (q, J=7.1Hz, ...
Embodiment 2
[0020] In a 250mL three-neck flask, dissolve lithium chloride (7.1g, 166.7mmol) and DBU (25.4g, 166.7mmol) in acetonitrile (50mL), and add triethylphosphonoacetic acid at 0°C under N2 protection The ester (37.4g, 166.7mmol) was stirred for 10min, raised to room temperature and stirred for 30min, then added 4-formylphenylboronic acid (5.0g, 33.3mmol), and reacted at room temperature for 3h. After the reaction is complete, pour the reaction solution into water, extract with ethyl acetate (50mL×3), combine the organic layers, dry over anhydrous sodium sulfate, concentrate under reduced pressure, add concentrated hydrochloric acid (2mL) and a solid is precipitated, suction filtered, and the filter cake Slurry with a small amount of n-hexane (5 mL) and dry to obtain 5.9 g of 4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid with a yield of 80.3%.
[0021] 1 H NMR (400MHz, DMSO) δ7.82(d, J=8.2Hz, 2H), 7.67(d, J=7.4Hz, 2H), 7.62(s, 1H), 6.66(d, J=16.1Hz, 1H ), 4.20 (q, J=7.1Hz, ...
Embodiment 3
[0023] In a 250mL three-neck flask, dissolve lithium chloride (1.4g, 33.3mmol) and DBU (5.1g, 33.3mmol) in acetonitrile (50mL), and add triethylphosphonoacetic acid at 0°C under N2 protection The ester (7.5g, 33.3mmol) was stirred for 10min, warmed up to room temperature and stirred for 30min, 4-formylphenylboronic acid (5.0g, 33.3mmol) was added, and reacted at room temperature for 8h. After the reaction is complete, pour the reaction solution into water, extract with ethyl acetate (50mL×3), combine the organic layers, dry over anhydrous sodium sulfate, concentrate under reduced pressure, add concentrated hydrochloric acid (2mL) and a solid is precipitated, suction filtered, and the filter cake Slurry with a small amount of n-hexane (5 mL) and dry to obtain 5.0 g of 4-(E-3-ethoxy-3-oxo-1-propen-1-yl)phenylboronic acid with a yield of 67.9%.
[0024] 1 H NMR (400MHz, DMSO) δ7.82(d, J=8.2Hz, 2H), 7.67(d, J=7.4Hz, 2H), 7.62(s, 1H), 6.66(d, J=16.1Hz, 1H ), 4.20 (q, J=7.1Hz, 2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com