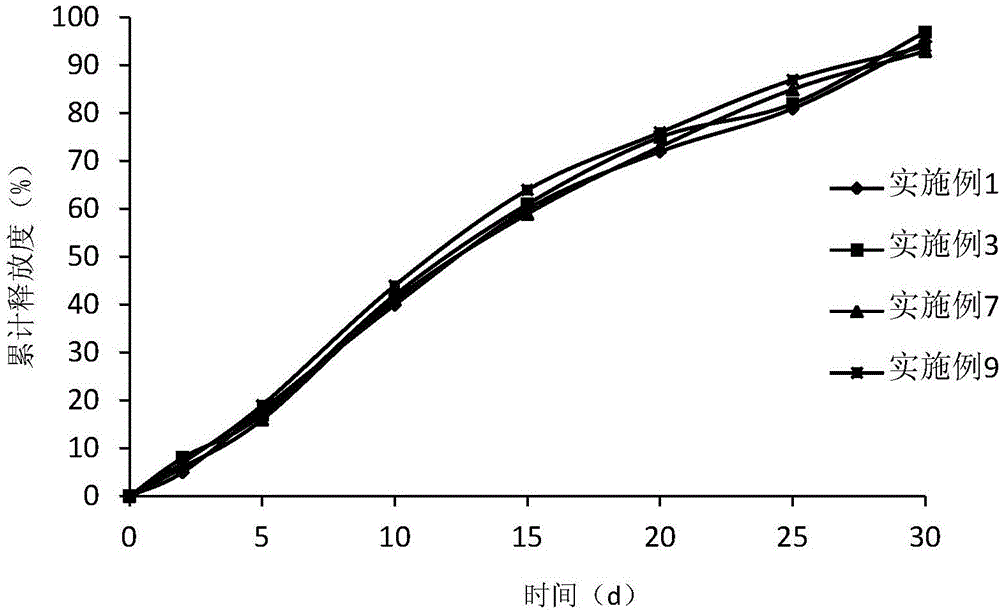
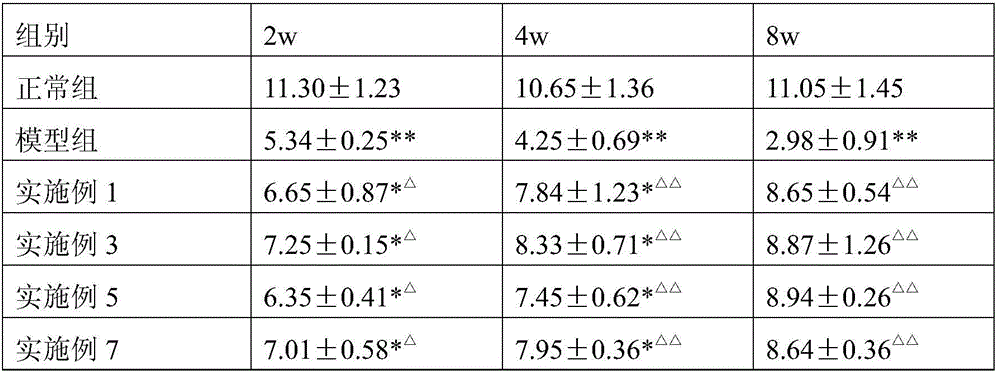
GLP-1 analogue and ziconotide composite slow-release microsphere preparation
A technology of sustained-release microsphere preparation and ziconotide, which is applied in the directions of drug combination, drug delivery, peptide/protein composition, etc., can solve the problems of undiscovered combination application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A preparation method of GLP-1 analog and ziconotide composition sustained-release microsphere preparation, comprising the steps of:
[0050] Step 1) adding water to the GLP-1 analogue and ziconotide to form a drug solution A; adding a biodegradable and biocompatible polymer material to form a solution B;
[0051] Step 2) The solution A and the solution B are mixed and ultrasonicated to form colostrum, and the colostrum is added to an aqueous stabilizer solution saturated with an organic mixed solvent, and homogeneously emulsified to obtain double emulsion;
[0052] Step 3) Stir the double emulsion at room temperature for 1 hour, then raise the temperature to 40°C-45°C and keep it for 1 hour, then lower the temperature to 10°C, add a freeze-drying protective agent, collect the particles by sieving, freeze-dry, and sterilize by radiation.
[0053] Preferably, the organic solvent in step 1) is selected from one of ethyl acetate, benzyl alcohol mixed solution or dichloromet...
Embodiment 1
[0060] Weigh 0.2 mg of liraglutide and 0.2 mg of ziconotide and add water to make a drug solution with a concentration of 40%, dissolve 1 g of polylactide-glycolide with 25 mL of ethyl acetate and 10 mL of benzyl alcohol, and mix the two Ultrasonic for 2 minutes, white homogeneous colostrum is formed. Colostrum was added into 1000 mL of 0.01% (weight to volume ratio) polyvinyl alcohol aqueous solution (containing 1% benzyl alcohol and 1% ethyl acetate) at 6°C through a syringe through a syringe at 1600 rpm, and homogeneously emulsified for 2 minutes to obtain double emulsion . Move the double emulsion to the cantilever mixer at a speed of 600rpm, stir for 1 hour, then raise the temperature to 40°C and keep it for 1 hour, then lower it to 10°C, add 0.1g of sorbitol to obtain the mixture, filter with a sieve, and freeze-dry to obtain powdery microspheres . Observed by a JSM-5600 scanning electron microscope, the pores of the microspheres are continuous and complete, and the mi...
Embodiment 2
[0062] Weigh 0.25 mg of exenatide and 0.3 mg of ziconotide and add water to make a drug solution with a concentration of 20%, dissolve 1 g of polyhydroxybutyrate valerate with 30 mL of ethyl acetate and 10 mL of benzyl alcohol, and mix the two Ultrasonic for 2 minutes, white homogeneous colostrum is formed. Colostrum was added into 1000 mL of 0.02% polyvinyl alcohol solution (containing 0.5% benzyl alcohol and 1% ethyl acetate) at 6°C through a syringe through a syringe at a homogeneous speed of 1600 rpm, and homogeneously emulsified for 2 minutes to obtain double emulsion. Move the double emulsion to a cantilever mixer at a speed of 600rpm, stir for 1 hour, then raise the temperature to 45°C and keep it for 1 hour, then lower it to 10°C, add 0.1g of lactose to obtain a mixture, filter through a sieve, and freeze-dry to obtain powdered microspheres. Observed by the JSM-5600 scanning electron microscope, the pores of the microspheres are continuous and complete, and the microsp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com