Preparation method of Ag@AgCl/TiO2-graphene oxide composite
A composite material and graphene technology, applied in chemical instruments and methods, water treatment of special compounds, water/sludge/sewage treatment, etc., can solve the problem of low quantum efficiency of photocatalysis, achieve good adsorption of pollutants, improve light Effects of catalytic efficiency and strong adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) First, 0.068g AgNO 3 and 0.5g PDDA were dissolved in 20ml deionized water, and then AgNO 3 Add the solution dropwise to the PDDA solution, and stir for 15 minutes to obtain the Ag+ dispersion;
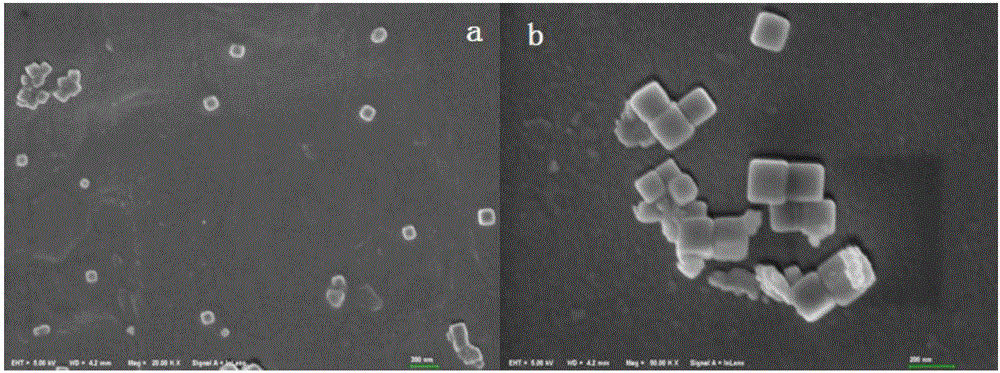
[0034] (2) Dissolve 0.0234g NaCl and 0.1g glucose in 20mL and 60mL deionized water respectively to prepare a solution, and add the prepared NaCl solution and glucose solution dropwise to the above Ag + In the dispersion liquid, after stirring for one hour, the mixed solution was transferred to a reaction kettle, heated to 180°C and kept for hydrothermal reaction for 24 hours, and centrifuged after the reaction was completed. The centrifuged product was washed three times with ethanol and deionized water respectively, and the washed product was dissolved in deionized water to obtain Ag@AgCl sol. When preparing the Ag@AgCl sol, the product was dissolved in 20ml of deionized water per 120ml of the reaction solution. from figure 1 -a and figure 1In -b, it can be seen that t...
Embodiment 2
[0042] The other steps and parameters of Examples 2-4 are the same as in Example 1, except that the dosage of Ag@AgCl sol in step (4) is 60mL (Example 2), 80mL (Example 3) and 100ml (Example 3) respectively. Example 4).
Embodiment 5
[0046] Other parameter steps of embodiment 5-7 are identical with embodiment 3, difference is: in step (5) TiO 2 The powders were used in amounts of 0.2 g (Example 5), 0.6 g (Example 6) and 1 g (Example 7), respectively.
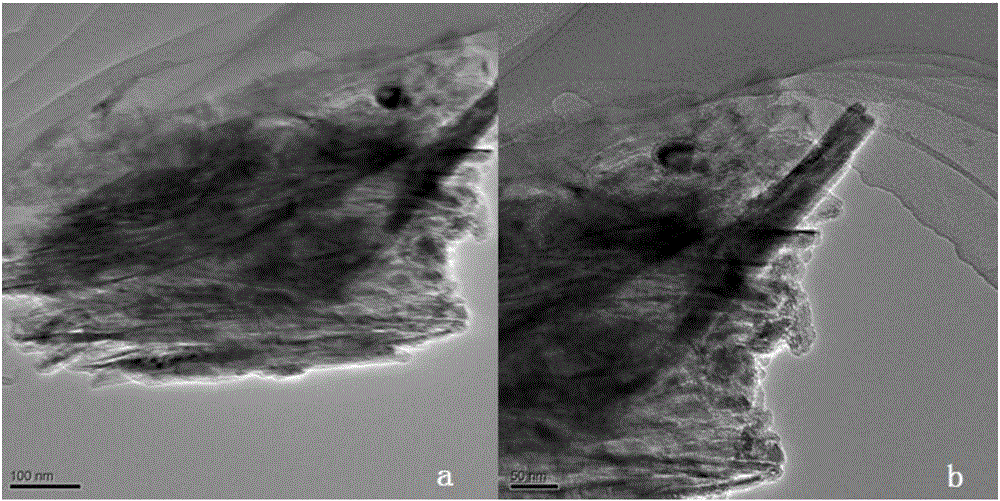
[0047] Compared Figure 4 with Figure 5 , when TiO 2 When the dosage is 0.2g, graphite oxide almost replaces TiO 2 The coating of nanotubes greatly affects its absorption of light; and when TiO 2 When the dosage is 1g, TiO 2 Excess, some TiO 2 Not formed into tubes and not composited with graphite oxide.
[0048] Then compare its photocatalytic performance, when TiO 2 When the dosage is 0.4g, the photocatalytic performance is the best. Within 20 minutes, the photocatalytic efficiency reaches over 80%, and the methylene blue is basically degraded in about 40 minutes. When TiO 2 When the dosage was 0.6g, the photocatalytic performance decreased. And when TiO 2 When the dosage is 0.2g and 1g, the photocatalytic performance drops sharply. The former ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com