Method for preparing porous Fe3O4 (ferroferric oxide) magnetic nanometer microspheres by one-step method and application thereof
A technology of ferroferric oxide and magnetic nanometers, which is applied in the direction of chemical instruments and methods, iron oxide/iron hydroxide, ferrous oxide, etc., can solve the problems of complex preparation process and time-consuming, and achieve simple preparation process , good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
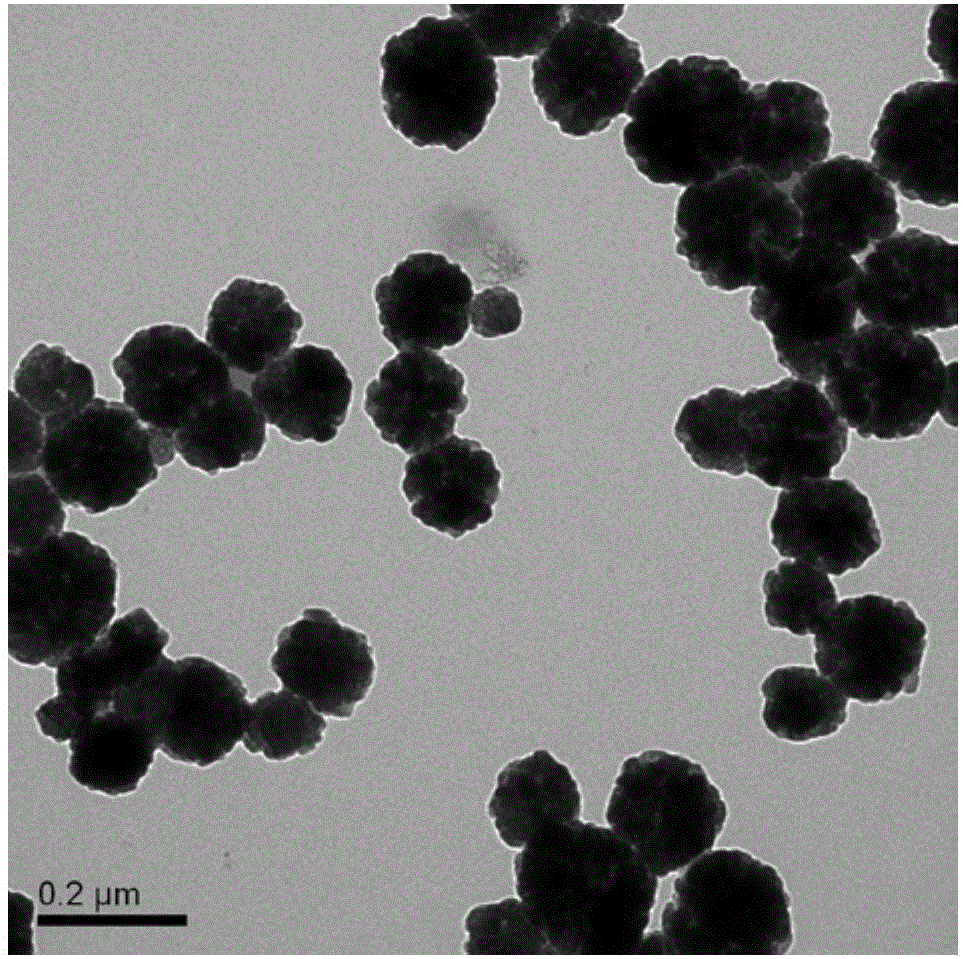
[0021] Take 2.5g FeCl 3 ·6H 2 O, 3.6g NaAc and 4.8g polyethyleneimine (molecular weight 10000) were respectively dissolved in three parts of ethylene glycol, and then the three solutions were mixed (72.5 g of ethylene glycol in total), and a uniform solution was formed after thorough stirring. Put it in a high-pressure reactor and react at 180°C for 24h, collect the product with a magnet, wash it with water and absolute ethanol three times, and dry it in vacuum at 50°C for 24h to obtain polyethyleneimine-modified Fe 3 o 4 Magnetic porous nanospheres. The saturation magnetization of the magnetic microspheres is 74.6emu / g, and the content of polyethyleneimine is 9.4% by thermogravimetric analysis; the specific surface area is 78.5m2 by nitrogen adsorption instrument. 2 / g. Take 0.02g Fe 3 o 4 Microspheres were added to 50mL copper ion wastewater (pH=5.5, Cu 2+ Concentration is 50mg / L), magnetically separated after shaking at 25°C for 2h, Cu 2+ The removal rate reaches 98...
Embodiment 2
[0023] Take 2.0g FeCl 3 ·6H 2 O, 8.0g NaAc and 3.0g polyethyleneimine (molecular weight 10000) were dissolved in three parts of ethylene glycol respectively, then the three solutions were mixed (72.5g of ethylene glycol in total), and a uniform solution was formed after thorough stirring. Put it in a high-pressure reactor and react at 200°C for 6h, collect the product with a magnet, wash it with water and absolute ethanol three times, and dry it in vacuum at 50°C for 24h to obtain polyethyleneimine-modified Fe 3 o 4 Magnetic porous nanospheres. The saturation magnetization of the magnetic microspheres is 75.2emu / g, and the content of polyethyleneimine is 7.5% by thermogravimetric analysis; the specific surface area is 57.7m2 by nitrogen adsorption instrument. 2 / g. Take 0.02g Fe 3 o 4 Microspheres were added to 50mL copper ion wastewater (pH=5.5, Cu 2+Concentration is 50mg / L), magnetically separated after shaking at 25°C for 2h, Cu 2+ The removal rate reaches 95.6%. A...
Embodiment 3
[0025] Take 2.0g FeCl 3 ·6H 2 O, 8.0g NaAc and 0.85g polyethyleneimine (molecular weight 1800) were dissolved in three parts of ethylene glycol respectively, then the three solutions were mixed (72.5g of ethylene glycol in total), and a uniform solution was formed after thorough stirring. Put it in a high-pressure reactor and react at 220°C for 2 hours, collect the product with a magnet, wash it with water and absolute ethanol three times, and dry it in vacuum at 50°C for 24 hours to obtain polyethyleneimine-modified Fe 3 o 4 Magnetic porous nanospheres. The saturation magnetization of the magnetic microspheres is 67.9emu / g, and the content of polyethyleneimine is 2.3% by thermogravimetric analysis; the specific surface area is 28.3m2 by nitrogen adsorption instrument. 2 / g. Take 0.04g Fe 3 o 4 Add microspheres in 50mL lead ion wastewater (pH=5.5, Pb 2+ Concentration is 35mg / L), magnetically separated after shaking at 25°C for 2h, Pb 2+ The removal rate reaches 96.3%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com