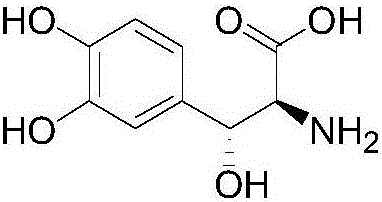
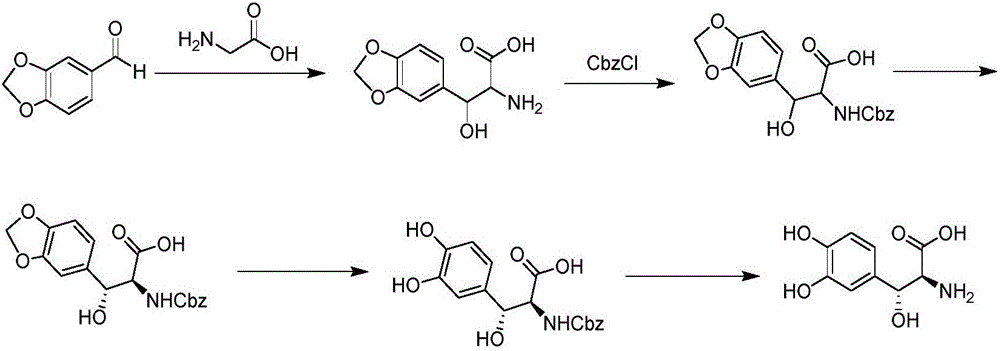
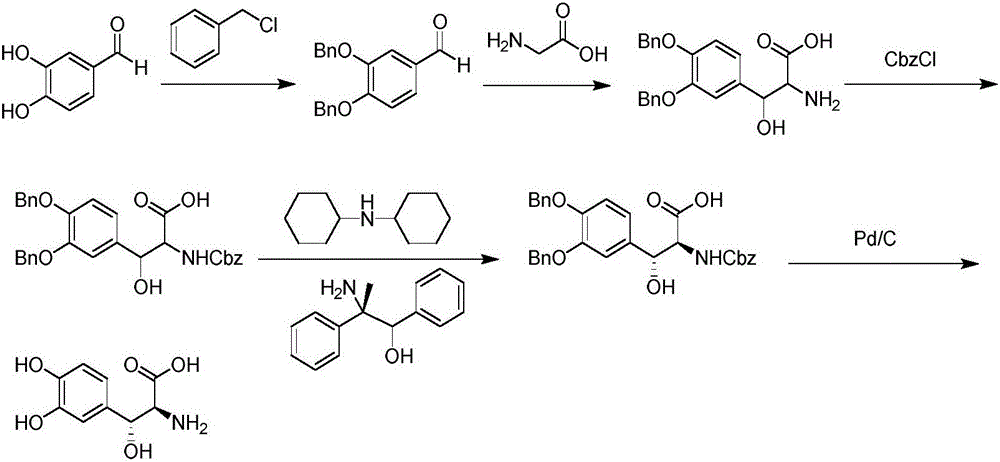
Preparation method of droxidopa
A technology of droxidopa and dibenzyloxybenzaldehyde, applied in the field of pharmaceutical synthesis, can solve the problems of low resolution yield, long reaction steps, difficulty in commercial purchase, etc., and achieves high total reaction yield and short reaction steps. , the effect of high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The method of the present invention will be described below through specific examples to make the technical solution of the present invention easier to understand and grasp, but the present invention is not limited thereto. The experimental methods described in the following examples, unless otherwise specified, are conventional methods; the reagents and materials, unless otherwise specified, can be obtained from commercial sources.
[0038] A preparation method of droxidopa, comprising the steps of:
[0039] S1. Using 3,4-dihydroxybenzaldehyde as a raw material, protecting the hydroxyl group with benzyl chloride to obtain 3,4-dibenzyloxybenzaldehyde;
[0040] S2. The 3,4-dibenzyloxybenzaldehyde and glycine prepared in S1 are subjected to an asymmetric condensation reaction with an enzyme as a catalyst in a buffer solution with a pH of 7.5 to 8.5, and the intermediate L- threo-3-(3,4-dibenzyloxyphenyl)serine.
[0041] Specifically, said S2 includes the following steps...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com