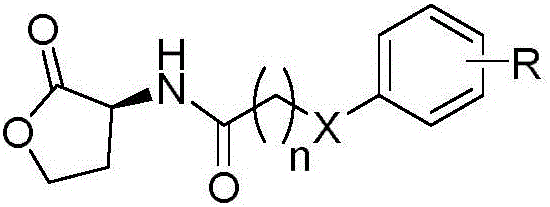
Benzene ring substituted N-acyl homoserine lactone compounds as well as preparation method and application thereof
A technology for acyl homoserine and ester compounds, which is applied in the field of medicinal chemistry and achieves the effects of simple preparation method, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
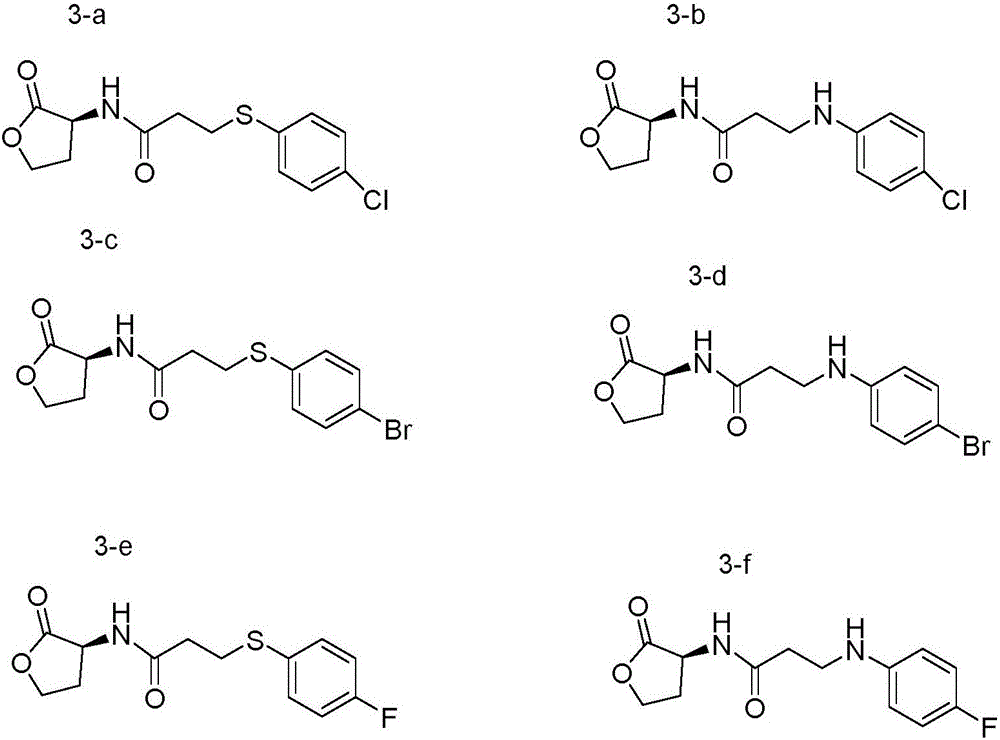
[0027] Embodiment 1: prepare derivative (3-a) shown in general formula 3
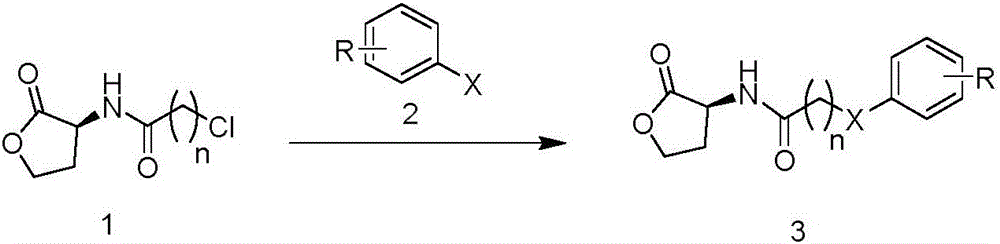
[0028] Compound (S)-2-chloro-N-(2-carbonyltetrahydrofuran-3-yl)propionamide (150mg, 0.79mmol), potassium carbonate (109mg, 0.79mmol) was dissolved in 10mL of acetonitrile, heated to 80°C , added p-chlorothiophenol (114mg, 0.79mmol), after constant temperature reaction for 4h, TLC tracked the completion of the reaction, suction filtered, removed potassium carbonate, evaporated the solvent under reduced pressure, and separated by silica gel column chromatography (eluent: ethyl acetate / Petroleum ether=1 / 1) to obtain 183 mg of off-white solid, namely compound (3-a), with a yield of 78%.
Embodiment 2
[0029] Embodiment 2: prepare derivative (3-b) shown in general formula 3
[0030] Compound (S)-2-chloro-N-(2-carbonyltetrahydrofuran-3-yl)propionamide (150mg, 0.79mmol), potassium carbonate (109mg, 0.79mmol) was dissolved in 10mL of acetonitrile, heated to 80°C , added p-chloroaniline (101mg, 0.79mmol), after constant temperature reaction for 7h, TLC tracked the completion of the reaction, suction filtered, removed potassium carbonate, evaporated the solvent under reduced pressure, and separated by silica gel column chromatography (eluent: ethyl acetate / petroleum Ether=1 / 1) to obtain 165 mg of off-white solid, namely compound (3-b), with a yield of 74%.
Embodiment 3
[0031] Embodiment 3: Preparation of derivatives shown in general formula 3 (3-c, 3-d, 3-e, 3-f)
[0032] Compound 3-c was prepared by the method described in Example 1 with p-bromothiophenol instead of p-chlorothiophenol.
[0033] Compound 3-d was prepared by the method described in Example 1 by substituting p-bromoaniline for p-chloroaniline.
[0034] Compound 3-e was prepared by the method described in Example 1 with p-fluorothiophenol instead of p-chlorothiophenol.
[0035] Using p-fluoroaniline instead of p-chloroaniline, compound 3-f was prepared by the method described in Example 1.
[0036] The chemical structure of the partial preferred compound that the present invention synthesizes, nuclear magnetic data is as follows table 1:
[0037]
[0038]
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com