Preparation process of halcinonide intermediate
A technology of hascinide and preparation process, applied in the field of chemical pharmacy, can solve the problems of long production cycle, difficult waste water treatment, increased environmental protection pressure, etc., and achieve the effects of simplifying production steps, shortening production cycle, and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
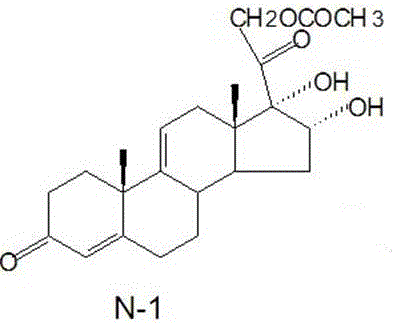
[0025] Embodiment 1: the preparation of intermediate N-3
[0026] A: Preparation of intermediate N-1 from triene acetate;
[0027] Add 8.1g of potassium permanganate to 105ml of water, heat up to 40°C, stir to dissolve the potassium permanganate, add 300ml of acetone after dissolving, stir and cool down to -15-20°C, set aside. Add 9g of sodium sulfite to 75ml of water, stir to dissolve, and set aside.
[0028] Add 15g of triene acetate to 600ml of acetone, cool down to -5-0°C, add 5.4ml of formic acid, stir for 5min, add the prepared potassium permanganate solution, control the temperature at -5-0°C for 10min, add the prepared A good sodium sulfite solution was heated up to 40°C, filtered, the filtrate was concentrated under reduced pressure to free of acetone, cooled to below 10°C, centrifuged, and dried at 60°C for 12 hours to obtain intermediate N-1, 14.5g, chromatographic purity: 98.2 %, yield 96.7%;
[0029] B: Preparation of Intermediate N-2 from Intermediate N-1;
...
Embodiment 2
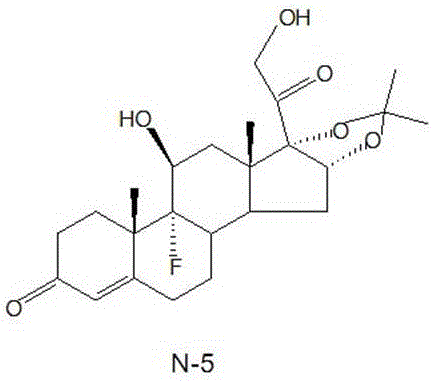
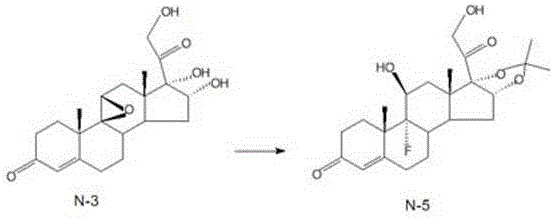
[0033] Embodiment 2: the preparation of intermediate N-5
[0034] Dissolve 130g of potassium carbonate in 400ml of water, stir to dissolve, and set aside. Add 50ml of hydrofluoric acid (70% HF) and 5ml of acetone into a 100ml plastic bottle, stir evenly, cool down to -40--30°C, control the temperature within -40--30°C, add 10g of the substrate within 1h Body N-3, after adding, react at temperature -35--30°C for 5 hours, return to -5-0°C, react for 10 minutes, slowly add the reaction solution into the prepared potassium carbonate solution, control the temperature at 10°C After that, the addition was completed within 1 hour, the pH value was adjusted to 7.0-7.5, the material was filtered out, and after drying, 10.35 g of the crude intermediate N-5 was obtained, with a chromatographic purity of 96.2%.
Embodiment 3
[0035] Embodiment 3: Preparation of Intermediate N-5
[0036] Dissolve 130g of potassium carbonate in 400ml of water, stir to dissolve, and set aside. Add 50ml of hydrofluoric acid (70% HF) and 10ml of acetone into a 100ml plastic bottle, stir evenly, cool down to -40--30°C, control the temperature within -40--30°C, add 10g of intermediates within 1 hour Substance N-3, after adding, react at temperature -35--30°C for 5 hours, return to -5-0°C, react for 10 minutes, slowly add the reaction solution into the prepared potassium carbonate solution, control the temperature at 10°C After that, the addition was completed within 1 hour, the pH value was adjusted to 7.0-7.5, the material was filtered out, and after drying, 10.41 g of intermediate N-5 crude product was obtained, and the chromatographic purity was 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com