Morphological control method of metal oxide/carbon negative electrode material for lithium ion battery
A technology for lithium-ion batteries and carbon anode materials, applied in battery electrodes, negative electrodes, secondary batteries, etc., can solve the problems of regulating MOF precursors, etc., and achieve the effects of convenient operation, simple process, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
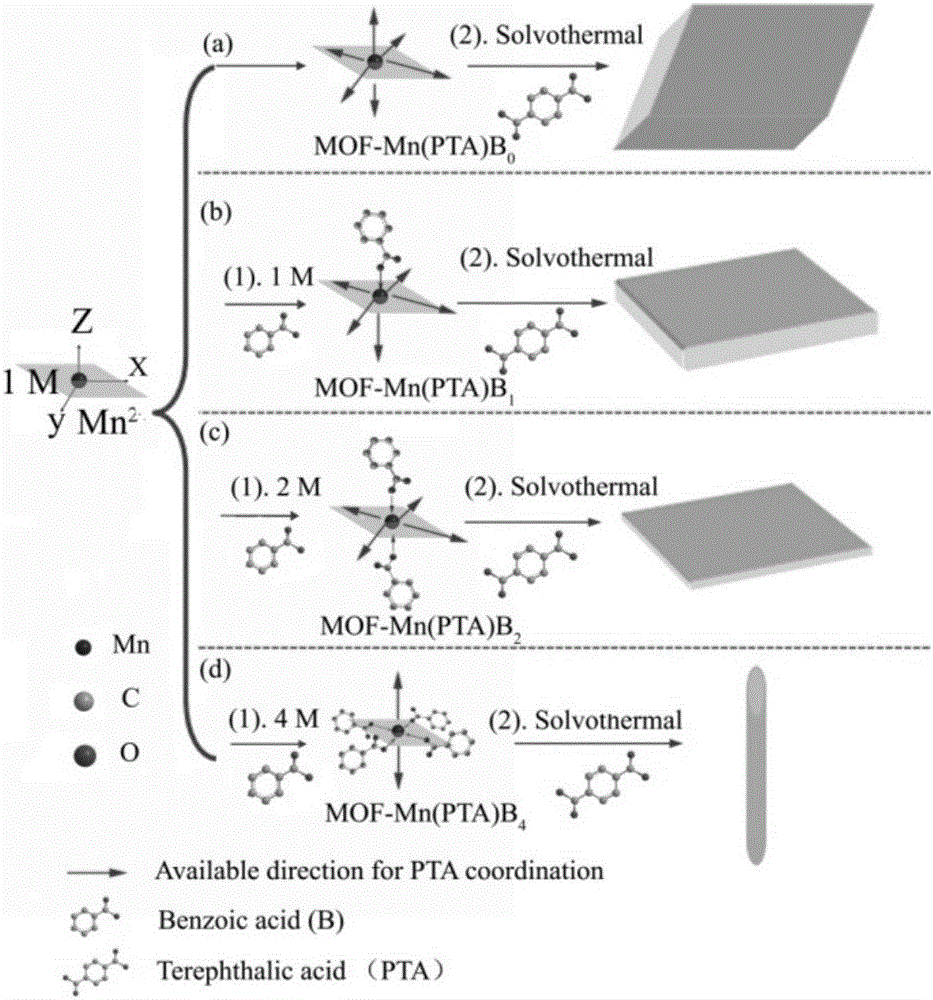
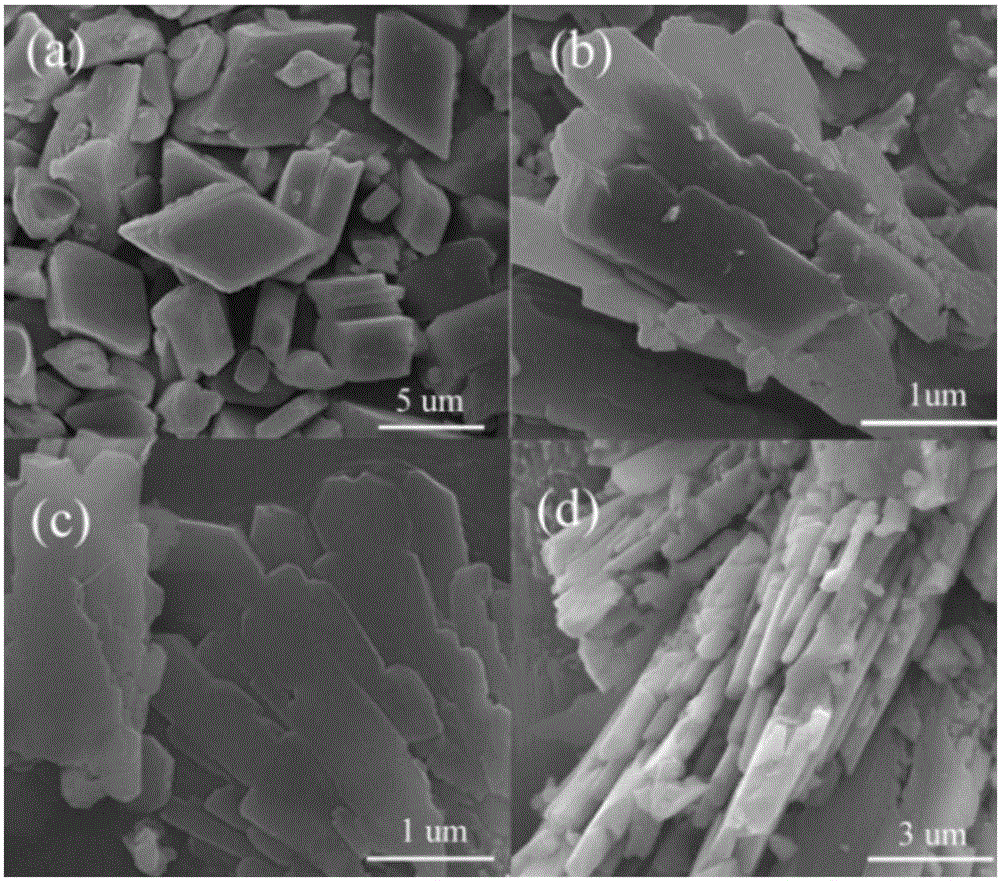
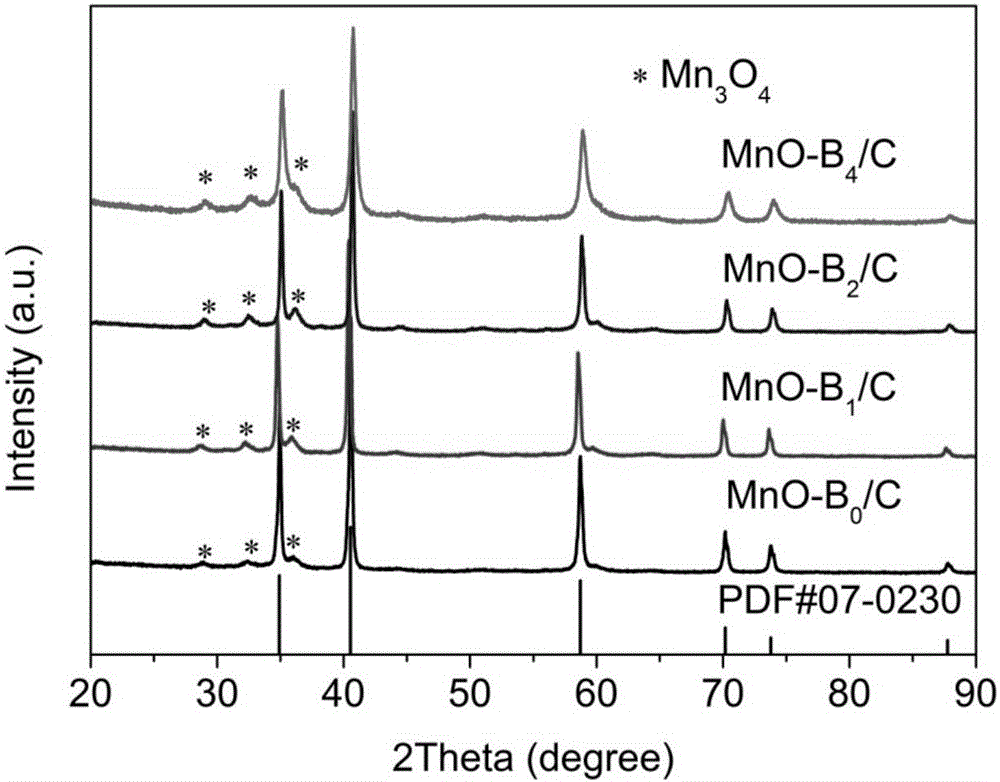
[0031] Example 1: Weigh 1.354g of manganese acetate and 2.75g of terephthalic acid, dissolve them in 50ml of dimethylformamide (in DMF), transfer them into a 100ml hydrothermal reactor, and heat and stir at 180°C for 10h. The reacted product was centrifuged, washed twice with absolute ethanol and deionized water, dried in an oven, and ground to obtain a white MOF precursor, which was designated as MOF-Mn(PTA)B 0 . The above white precursor was placed in a tube furnace filled with Ar gas flow, calcined at 600 °C for 2 h, and then naturally cooled to room temperature to obtain MnO-B 0 / C material. figure 1 a Recorded precursor MOF-Mn(PTA)B 0 SEM image, you can see MOF-Mn(PTA)B 0 It is a parallelepiped shape with a size of about 5 μm. figure 2 MnO-B 0 / C's XRD pattern, it can be seen from the figure that the material phase is mainly MnO, and contains a small amount of Mn 3 o 4 Miscellaneous. Table 1 records its BET specific surface area test results, it can be seen that ...
Embodiment 2
[0034] Weigh 1.354g of manganese acetate and 0.674g of benzoic acid and dissolve them in 50ml of DMF. After stirring overnight, add 2.018g of terephthalic acid, transfer to a 100ml hydrothermal reactor, and heat and stir at 180°C for 10h. The reacted product was centrifuged, washed twice with absolute ethanol and deionized water, dried in an oven, and ground to obtain a white MOF precursor, which was designated as MOF-Mn(PTA)B 1 . The above white precursor was placed in a tube furnace, calcined at 600 °C for 2 h in an argon atmosphere, and then cooled naturally to room temperature to obtain MnO-B 1 / C. Precursor MOF-Mn(PTA)B 1 SEM such as figure 1 As shown in b, it can be seen that MOF-Mn(PTA)B 1 It is in the shape of a microsheet, with a length of 3 μm and a width of 0.8 μm. figure 2 MnO-B 1 / C's XRD pattern, it can be seen from the figure that the material phase is mainly MnO, and contains a small amount of Mn 3 o 4 Miscellaneous. See Example 1 for battery assembly...
Embodiment 3
[0036] Weigh 1.354g of manganese acetate and 1.348g of benzoic acid and dissolve in 50ml of DMF. After stirring overnight, add 1.834g of terephthalic acid, transfer to a 100ml hydrothermal reactor, and heat and stir at 180°C for 10h. The reacted product was centrifuged, washed twice with absolute ethanol and deionized water, dried and ground to obtain a white MOF precursor, which was designated as MOF-Mn(PTA)B 2 . The obtained white precursor was placed in a tube furnace filled with Ar gas, calcined at 600 °C for 2 h, and then naturally cooled to room temperature to obtain a MnO / C material which was designated as MnO-B 2 / C. figure 1 c records the precursor MOF-Mn(PTA)B 2 SEM image, you can see MOF-Mn(PTA)B 2 It is in the form of microsheets, with a length of about 3 μm and a width of about 0.9 μm, but the thickness is significantly smaller than that of MOF-Mn(PTA)B 1 . figure 2 MnO-B 2 / C XRD pattern, it can be seen that the material phase is mainly MnO, and contains a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com