Zr-MOF structure based CO selective methanation Ni/ZrO2 catalyst and preparation method thereof
A catalyst and selective technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of insufficient activity and selectivity stability, and achieve the effects of excellent CO methanation activity, low price and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Preparation of metal-organic framework material Zr-MOF: ZrCl 4 , BDC and DMF were uniformly mixed at a molar ratio of 1:1:180, crystallized at 120°C for 24 h, centrifuged, washed with DMF and methanol three times in turn, and dried at 65°C to obtain a white powder; the obtained white powder was washed with methanol solvent Soak for three days, and change the methanol every day to replace the remaining ligands and solvent molecules in the sample, and dry the resulting white powder at 65°C to obtain the metal-organic framework material Zr-MOF;
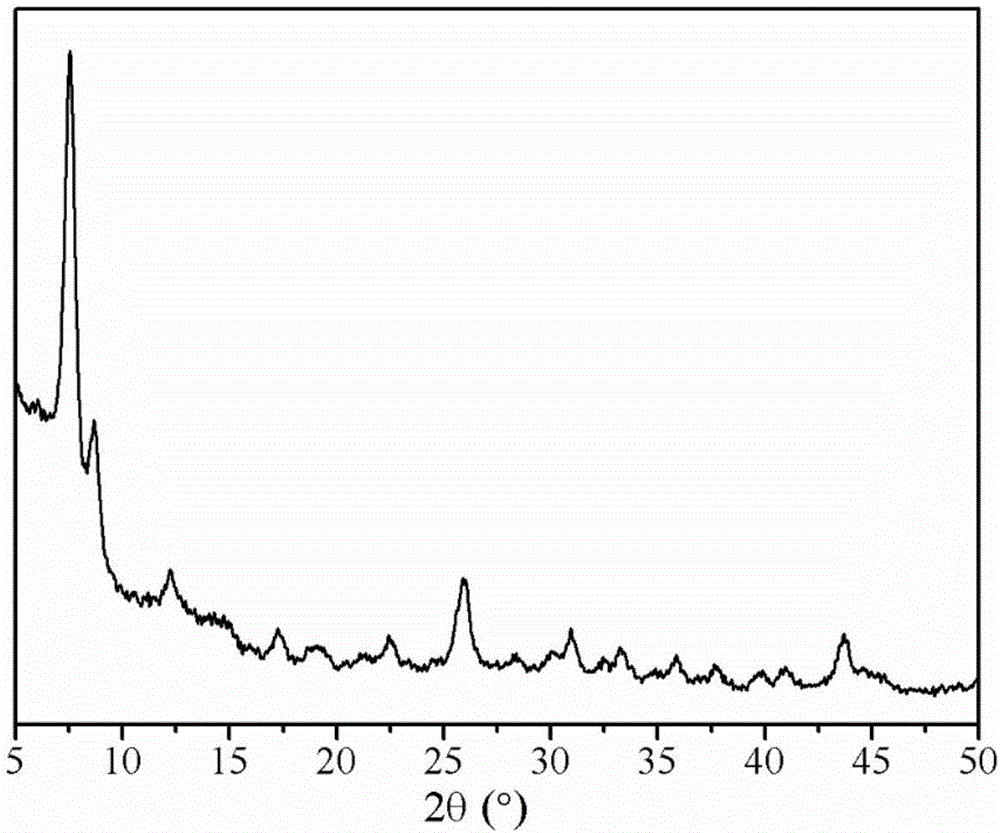
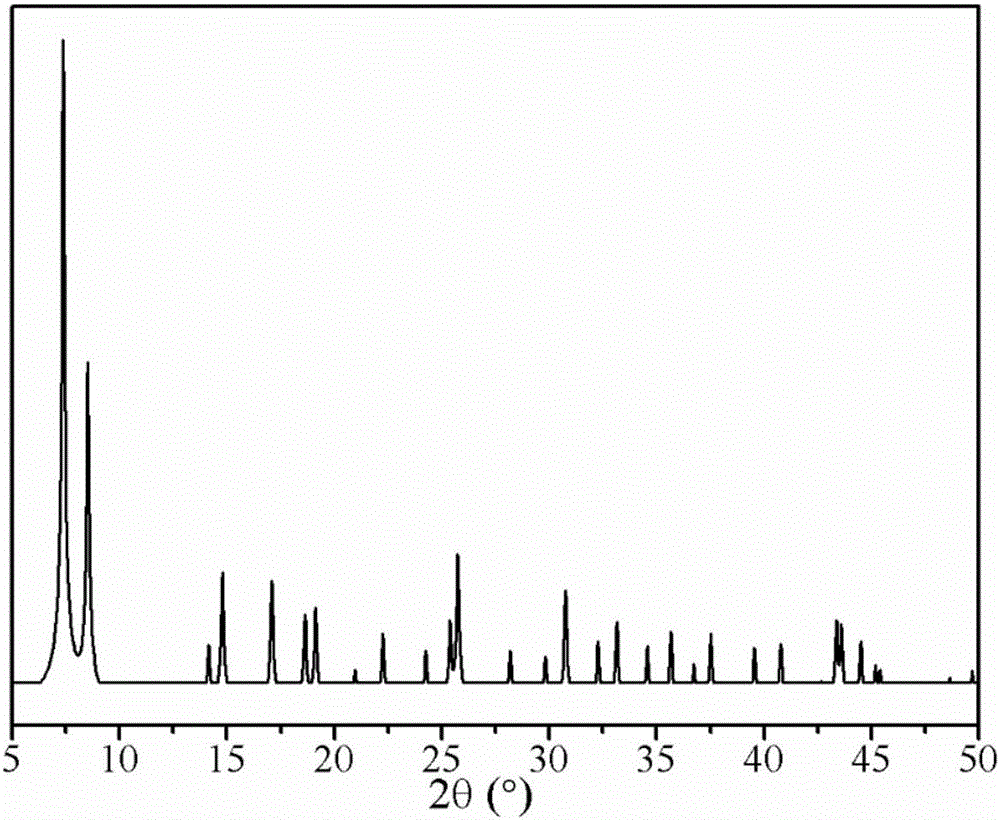
[0045] The XRD pattern of the prepared metal-organic framework material Zr-MOF and the XRD pattern obtained from its single crystal data simulation are as follows figure 1 and figure 2 As shown, it can be seen from the figure that the main diffraction peaks of the synthesized Zr-MOF are basically consistent with the XRD diffraction peaks obtained by the single crystal data simulation, indicating that the Zr-MOF was successfu...
Embodiment 2
[0053] (1) ZrCl 4 , BDC and DMF were uniformly mixed at a molar ratio of 1:1:150, crystallized at 110°C for 36 h, centrifuged, washed with DMF and methanol three times in turn, and dried at 70°C to obtain a white powder; the obtained white powder was washed with methanol solvent Soak for three days, and change methanol every day to replace the remaining ligands and solvent molecules in the sample, and dry the resulting white powder at 70°C to obtain the metal-organic framework material Zr-MOF;
[0054] (2) Take 8 ml concentration to be 6×10 -4 g / ml NiCl 2 ·6H 2 The aqueous solution of O was stirred evenly, and 0.2 g of pretreated Zr-MOF was added, stirred vigorously at room temperature for 12 h, centrifuged, dried at 70 °C, and then calcined in a muffle furnace at 550 °C for 2 h, 20% H 2 -N 2 Methanated Ni / ZrO can be obtained after reduction at 450°C for 2 hours in a mixed gas atmosphere 2 catalyst.
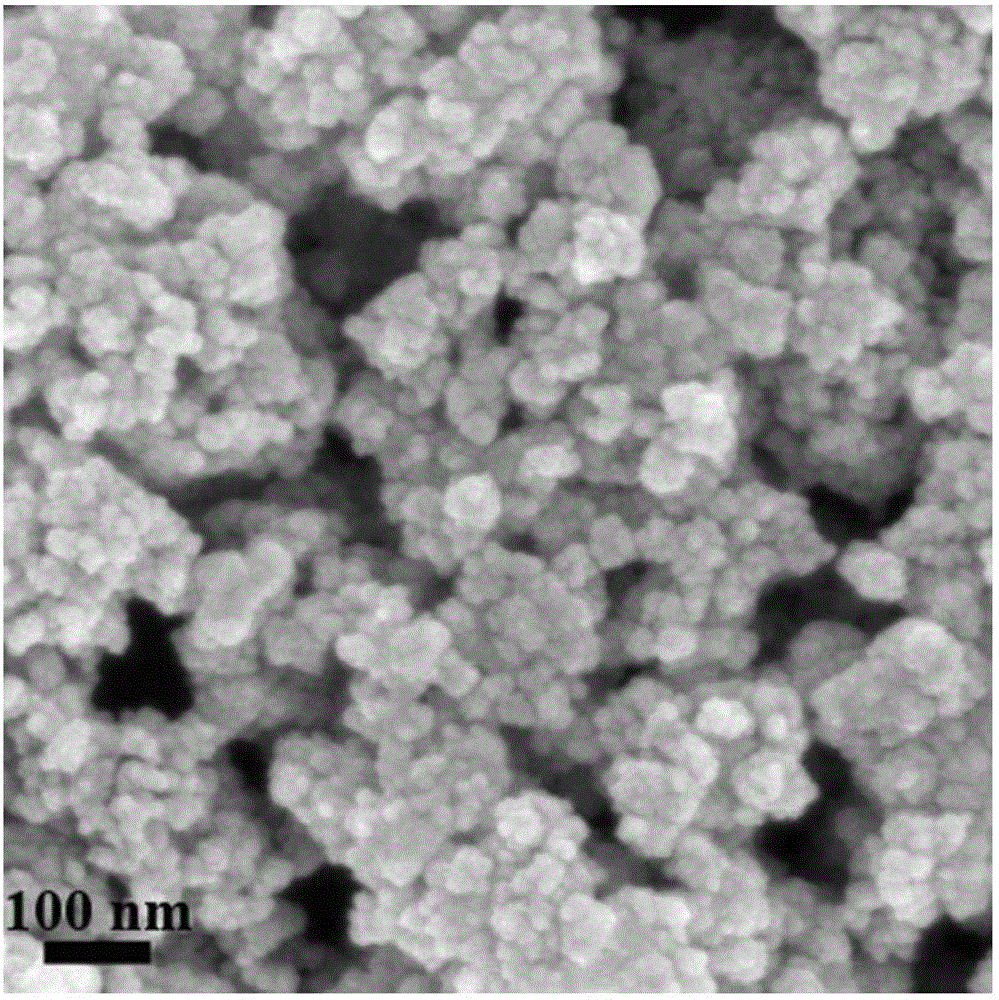
[0055] Prepared Ni / ZrO 2 The specific surface area of the catalyst...
Embodiment 3
[0058] (1) ZrCl 4 , BDC and DMF were uniformly mixed at a molar ratio of 1:1:250, crystallized at 130°C for 72 h, centrifuged, washed with DMF and methanol three times in turn, and dried at 65°C to obtain a white powder; the obtained white powder was washed with methanol solvent Soak for three days, and change the methanol every day to replace the remaining ligands and solvent molecules in the sample, and dry the resulting white powder at 75°C to obtain the metal-organic framework material Zr-MOF;
[0059] (2) Take 8 ml concentration to be 6×10 -3 g / ml NiCl 2 ·6H 2 O in ethanol solution, stirred evenly, added 0.2 g of pretreated Zr-MOF, stirred vigorously for 5 h, centrifuged, dried at 75 °C, and roasted in a muffle furnace at 450 °C for 4 h, 20% H 2 -N 2 After reduction at 350°C for 1 h in a mixed gas atmosphere, the methanated Ni / ZrO 2 catalyst.
[0060] Prepared Ni / ZrO 2 The specific surface area of the catalyst is 73.8 m 2 / g, the pore size is 15.9 nm, and the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com