Electrolytic liquor, electrolyte, polymer electrolyte as well as preparation method and application thereof
A technology of electrolyte and electrolyte, which is applied in the field of polymer electrolyte, electrolyte, and electrolyte, and can solve problems such as high cost, low conductivity, and complicated preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
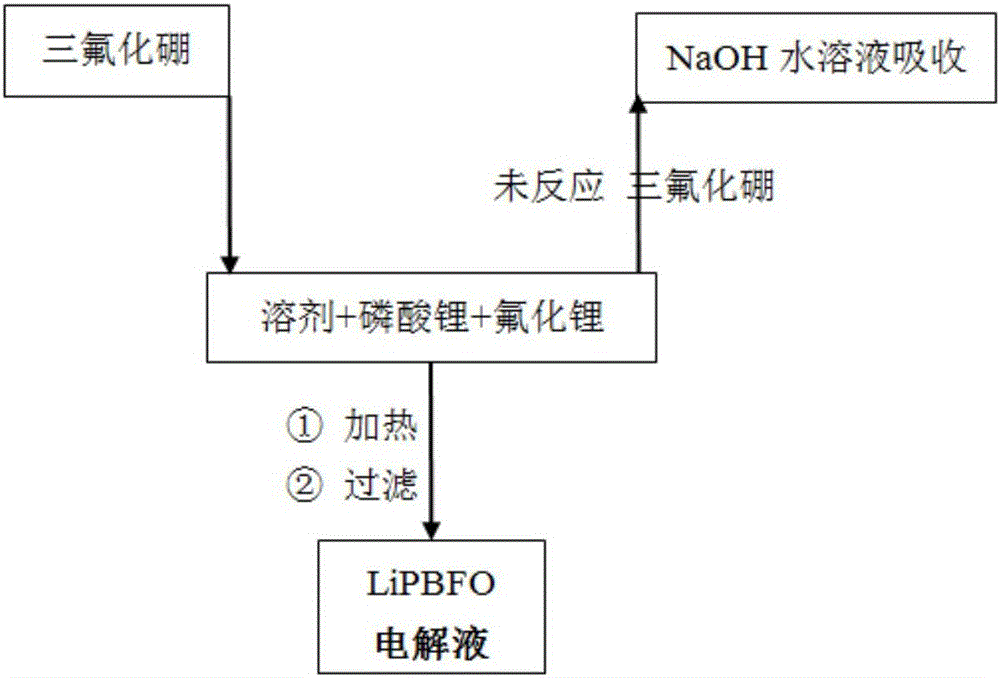
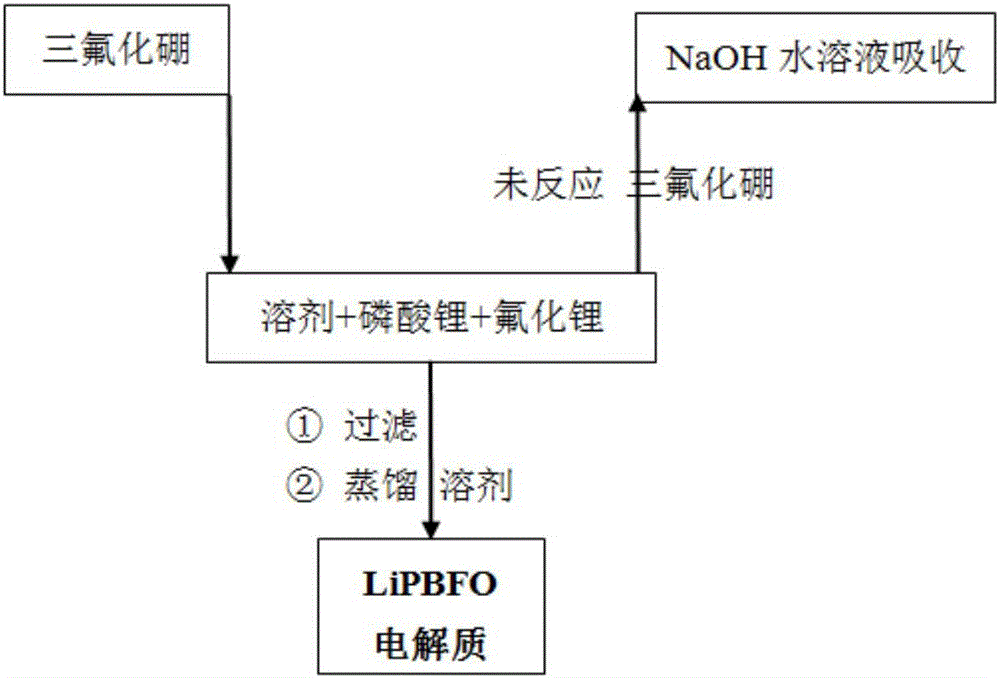
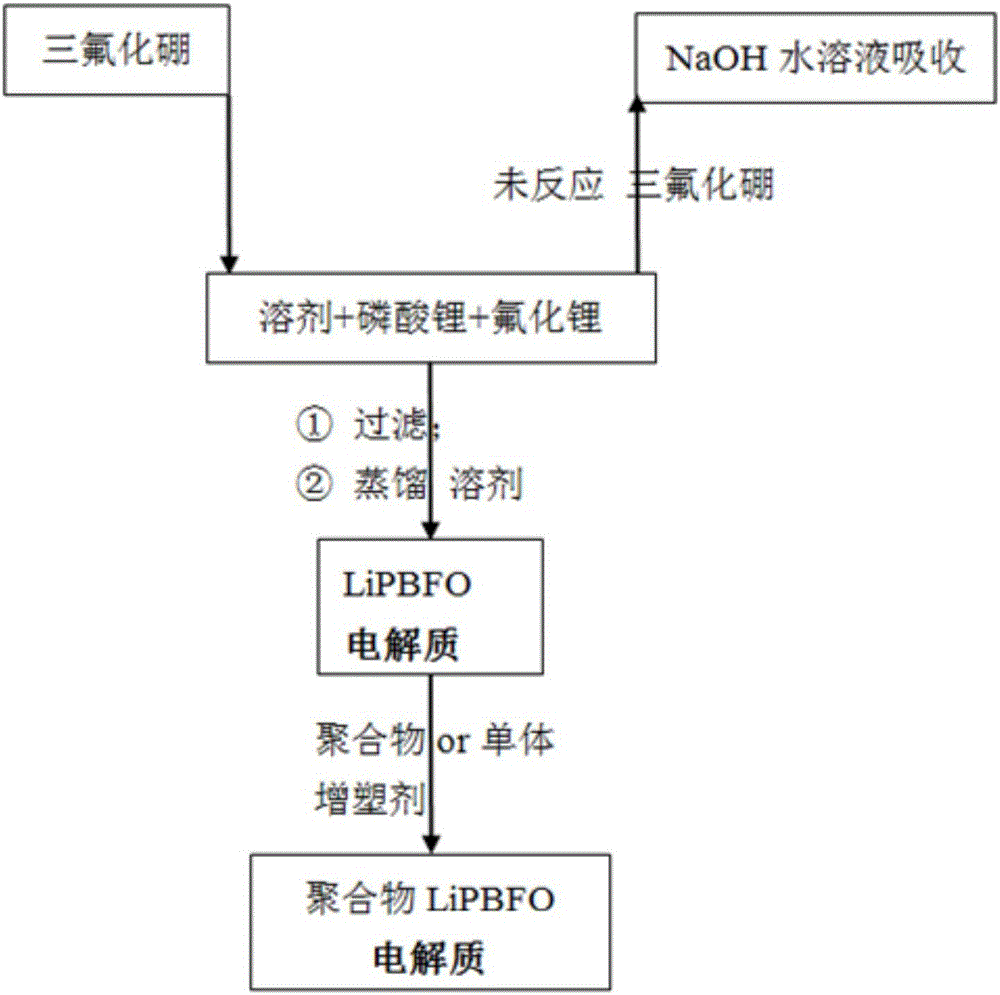
[0056] Add 402g of diethyl carbonate, 16.0g of lithium phosphate and 3.6g of lithium fluoride under the protection of nitrogen, stir at a temperature of 20 to 30°C, and after feeding 33.7g of boron trifluoride gas within 3 hours, continue at this temperature The mixture was stirred and reacted for 4 hours; the insoluble matter was removed by filtration, and the obtained filtrate was distilled off the solvent at a temperature of 60-70° C. by using a water pump to obtain 50.1 g of LiPBFO electrolyte. Its composition was determined to be 10.1% of boron content, 58.0% of fluorine content, 7.2% of lithium content, 8.0% of phosphorus content and 16.6% of oxygen content.
Embodiment 2
[0058] Under the protection of nitrogen, add 402g of diethyl carbonate, 16.0g of lithium phosphate and 7.2g of lithium fluoride, stir at a temperature of 20 to 30°C, and feed 33.7g of boron trifluoride gas within 2 hours, and continue to The reaction was stirred at high temperature for 6 hours; the insoluble matter was removed by filtration, and the obtained filtrate was distilled off the solvent by using a water pump under reduced pressure at a temperature of 60-70° C. to obtain 54.0 g of LiPBFO electrolyte. Its composition was measured as 9.4% of boron content, 59.0% of fluorine content, 8.4% of lithium content, 7.5% of phosphorus content and 15.5% of oxygen content.
Embodiment 3
[0060] Add 402g of diethyl carbonate, 16.0g of lithium phosphate and 10.7g of lithium fluoride under the protection of nitrogen, stir at a temperature of 20 to 30°C, and feed 51.9g of boron trifluoride gas within 5 hours. The reaction was stirred at high temperature for 3 hours; the insoluble matter was removed by filtration, and the obtained filtrate was distilled off the solvent by using a water pump at a temperature of 60-70° C. to obtain 75.4 g of LiPBFO electrolyte. Its composition was measured as 10.5% of boron content, 65.3% of fluorine content, 7.3% of lithium content, 5.4% of phosphorus content and 11.4% of oxygen content.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com