Azobenzene derivative as well as preparation method and application thereof
A technology of azobenzene derivatives and reaction, applied in the field of azobenzene derivatives and its preparation, to achieve the effects of reducing side effects, simple preparation method, and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
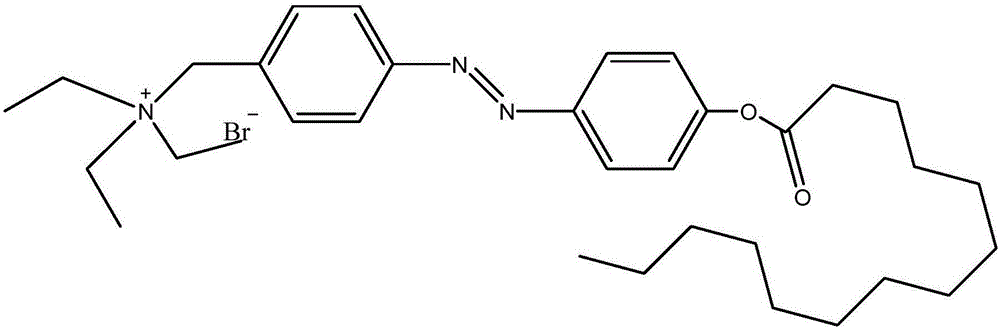
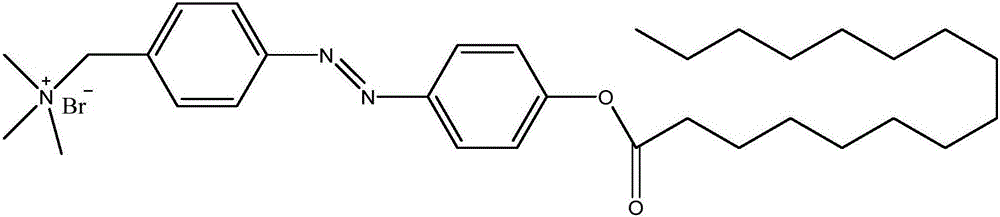
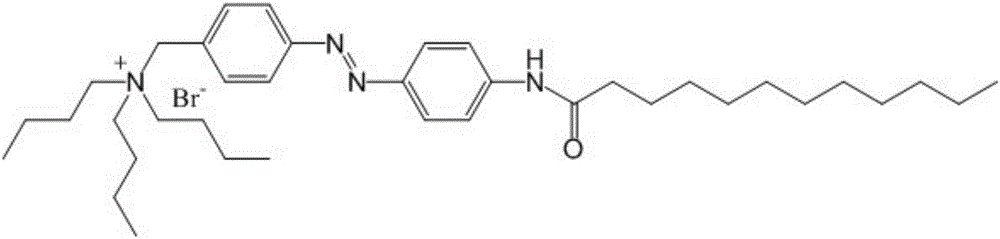
[0100] In the first specific preparation embodiment of the present invention, the structural formula of the azobenzene derivative is as figure 1 As shown, the preparation method of the azobenzene derivative includes the following steps:
[0101] S01. Make compound 1 (4-methyl-4' hydroxyazobenzene) and compound 2 (n-tetradecanoic acid) undergo a lipidation reaction under the catalysis and heating of concentrated sulfuric acid to generate compound 3 (4-methyl -4'Azophenyl n-tetradecanoate);
[0102] S02. Dissolve 4-methyl-4'-tetradecanoic acid azophenyl ester, N-bromosuccinimide and benzoyl peroxide in tetrachloromethane under argon atmosphere, and heat and reflux for reaction for 24 hours ;
[0103] S03. Cool the reaction system of step S02 to 0°C, and filter with suction to obtain powder;
[0104] S04. Wash the powder obtained in step S03 with ether to remove unreacted impurities to obtain compound 4 (4-bromomethyl-4'-tetradecanoic acid azophenyl ester);
[0105] S05. Dissolve 4-bromo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com