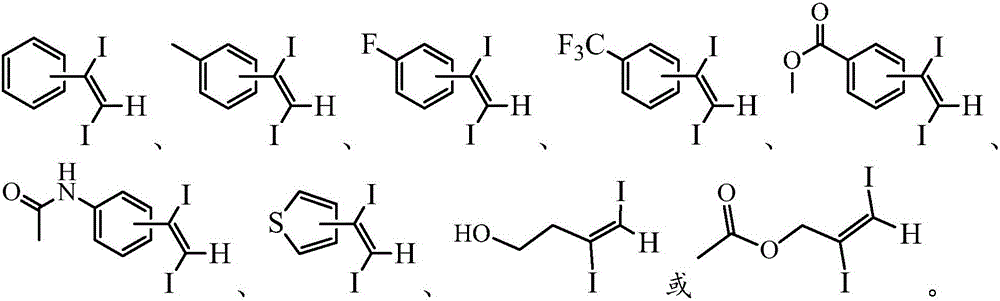
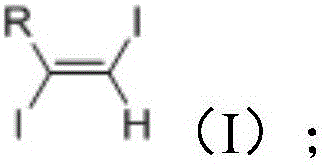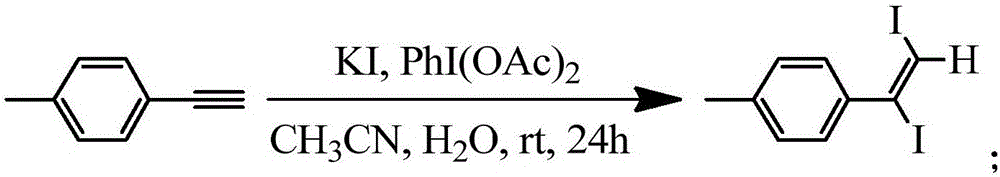Method for synthesizing 1, 2-diiodo-olefin compound in high-selectivity manner
A diiodoalkene, high-selectivity technology, applied in the field of synthetic chemistry, can solve the problems of using metal catalysts, harsh reaction conditions, and uncontrollable reactions, and achieve the effect of single product, mild reaction conditions, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Dissolve 38 μL (0.3 mmol) of p-methylphenylacetylene in 3 mL of acetonitrile, then add 3 mL of water and 124.5 mg (0.75 mmol) of potassium iodide, then add 96.6 mg (0.3 mmol) of iodobenzenediacetic acid to the reaction in batches within 30 min In the system, react at room temperature for 24 h, and then extract three times with ethyl acetate, combine the organic phases and concentrate under reduced pressure to obtain the crude product 1. The crude product 1 was separated and purified by silica gel column chromatography (n-hexane 100%) to obtain 111.9 mg of yellow liquid product 1 with a yield of 98%, and its NMR data were as follows:
[0036] 1 H NMR (400MHz, CDCl 3 , ppm): δ=7.26(d, J=8.0Hz, 2H), 7.22(s, 1H), 7.16(d, J=8.0Hz, 2H), 2.36(s, 3H);
[0037] 13 C NMR (100MHz, CDCl 3 , ppm): δ=140.2, 139.0, 129.1, 128.5, 96.6, 80.1, 21.4.
Embodiment 2
[0039]
[0040] Dissolve 33 μL (0.3 mmol) of phenylacetylene in 1 mL of acetonitrile, then add 3 mL of water and 124.5 mg (0.75 mmol) of potassium iodide, then add 96.6 mg (0.3 mmol) of iodobenzenediacetic acid to the reaction system in batches within 30 min, React at room temperature for 24 h, then extract three times with ethyl acetate, combine the organic phases and concentrate under reduced pressure to obtain the crude product 2. The crude product 2 was separated and purified by silica gel column chromatography (n-hexane 100%) to obtain 104.5 mg of white solid product 2 with a yield of 94%. The NMR data are as follows:
[0041] 1 H NMR (400MHz, CDCl 3 ,ppm):δ=7.26-7.36(m,5H),7.24(s,1H);
[0042] 13 C NMR (100MHz, CDCl 3 , ppm): δ=143.1, 129.0, 128.5, 128.4, 96.2, 80.8.
Embodiment 3
[0044]
[0045] Dissolve 34.4 μL (0.3 mmol) of 4-fluorophenylacetylene in 1 mL of acetonitrile, then add 3 mL of water and 124.5 mg (0.75 mmol) of potassium iodide, and then add 193.3 mg (0.6 mmol) of iodobenzenediacetic acid to the In the reaction system, react at room temperature for 12 h, then extract three times with ethyl acetate, combine the organic phases and concentrate under reduced pressure to obtain the crude product 3. The crude product 3 was separated and purified by silica gel column chromatography (n-hexane 100%) to obtain 108.8 mg of yellow liquid product 3 with a yield of 97%. The NMR data are as follows:
[0046] 1 H NMR (400MHz, CDCl 3 , ppm): δ=7.33-7.36(m, 2H), 7.26(s, 1H), 7.06(t, J=8.8Hz);
[0047] 13 C NMR (100MHz, CDCl 3 , ppm): δ=162.5 (d, J=248Hz), 139.1 (d, J=3.4Hz), 130.6 (d, J=8.5Hz), 115.6 (d, J=21.9Hz), 94.9, 81.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com