Method for preparing methyl acrylate
A technology of methyl acrylate and methyl acetate, which is applied in the field of preparation of chemical raw materials, and can solve problems such as low product yield and easy deactivation of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
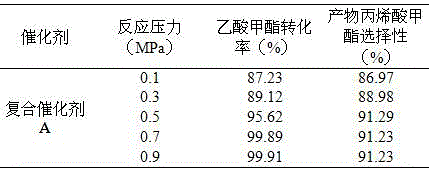
[0033] The mixed gas of paraformaldehyde and methyl acetate passed through the reactor loaded with 100 g of solid catalyst at a temperature of 360 °C and a pressure of 0.5 MPa. The reaction time is 100 h. The reaction results are shown in Table 1:
[0034] Table 1 Effect of different aldehyde-ester ratios on the conversion of methyl acetate
[0035]
[0036] It can be seen from Table 1 that as the molar ratio of aldehyde to ester increases, the conversion rate of methyl acetate also increases. When the molar ratio of aldehyde to ester is 1.2:1, the conversion rate of methyl acetate is 99.98%, which is close to complete conversion. When the molar ratio of aldehyde to ester (0.8:1, 0.9:1, 1:1) gradually increased, the selectivity of MA also gradually increased. When the molar ratio of aldehyde to ester was 1:1, the conversion rate of methyl acetate was 99.44%. When the molar ratio of aldehyde to ester (1.1:1, 1.2:1) gradually increased, the selectivity of MA decreased gradu...
Embodiment 2
[0038]A mixture of methylal, paraformaldehyde and methyl acetate (molar ratio 0.2:1:1) was passed through a reactor loaded with 100 g of solid catalyst at a pressure of 0.5 MPa. The reaction time was 100 h, and the reaction temperatures were 300, 320, 340, 360, 380, and 400 °C, respectively. The reaction results are shown in Table 2:
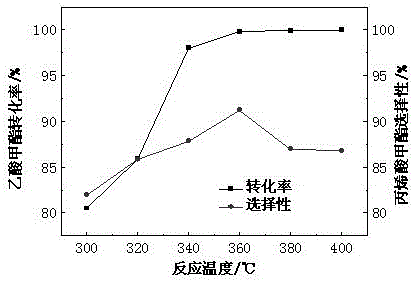
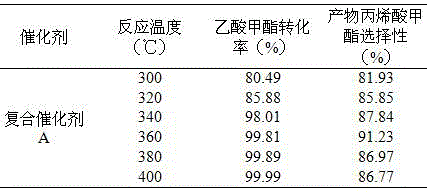
[0039] The influence of table 2 different reaction temperatures on methyl acetate conversion rate
[0040]
[0041] According to Table 2, it can be seen that with the increase of temperature, the conversion rate of methyl acetate increases continuously. When the reaction temperature is 400°C, the conversion rate of methyl acetate is 99.99%, which is close to complete conversion. When the temperature (300, 320, 340, 360 ℃) gradually increased, the selectivity of the main product MA gradually increased, and when the temperature was 360 ℃, the MA selectivity reached the maximum value of 91.23%. After that, the reaction temperature (380, 400°C)...
Embodiment 3
[0043] The mixed gas of methylal, tetrapolyoxymethylene and methyl acetate (molar ratio 0.2:1:1) was passed through the reactor loaded with 100 g of solid catalyst under the condition of 0.5 MPa. The reaction time was 100 h, and the reaction temperatures were 300, 320, 340, 360, 380, and 400 °C, respectively. The reaction results are shown in Table 3:
[0044] The influence of table 3 different reaction temperatures on the conversion rate of methyl acetate
[0045]
[0046] According to Table 3, it can be seen that with the increase of temperature, the conversion rate of methyl acetate increases continuously. When the reaction temperature is 400°C, the conversion rate of methyl acetate is 99.99%, which is close to complete conversion. When the temperature (300, 320, 340, 360 ℃) gradually increased, the selectivity of the main product MA gradually increased, and when the temperature was 360 ℃, the MA selectivity reached the maximum value of 91.21%. After that, the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com