Biphenyl bridged dinuclear iron complex and its preparation method and use method
A technology of iron complexes and biphenyl bridges, which is applied in the field of biphenyl bridged dinuclear iron complexes and its preparation, can solve the problems of low activity, complicated preparation process, and harsh preparation conditions, and improve the use performance and processing performance , Raw materials are cheap and easy to get, easy to separate and purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
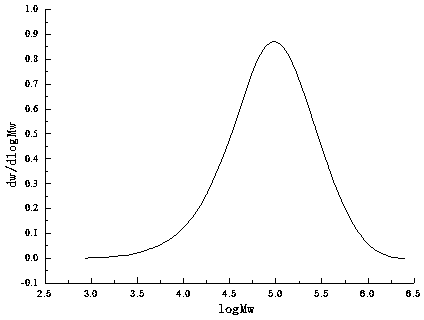
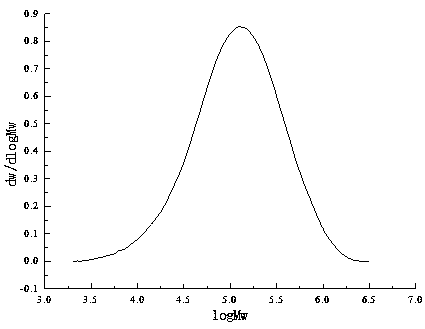
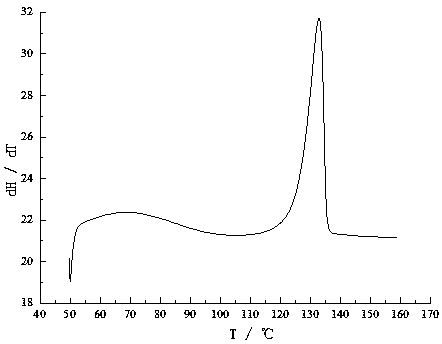
Image
Examples
Embodiment 1
[0039] A biphenyl bridged dinuclear iron complex and a preparation method thereof. The described biphenyl bridged dinuclear iron complex is denoted as D 1 , whose structural formula is:
[0040]
[0041] Biphenyl bridged dinuclear iron complex D 1 The concrete steps of preparation method are:
[0042] Step 1. Ligand L 1 preparation of
[0043] Ligand L 1 The structural formula is:
[0044]
[0045] Ligand L 1 Preparation method: Dissolve 10mmol benzidine and 20mmol salicylaldehyde in 3mL tetrahydrofuran, stir to dissolve, then add 0.5mL formic acid, and react at 60°C for 6h to obtain reaction system Ⅰ. Then the reaction system I was cooled to 0°C, and a yellow powder was precipitated, which was filtered by suction to obtain the ligand L 1 crude products. Then it was recrystallized with a mixed solvent of 2mL tetrahydrofuran and 2mL ethanol, and filtered with suction to obtain yellow needle-like crystals. Vacuum drying at 70°C yielded 3.03 g of Ligand L 1 , and...
Embodiment 2
[0053] A biphenyl bridged dinuclear iron complex and a preparation method thereof. The described biphenyl bridged dinuclear iron complex is denoted as D 2 , whose structural formula is:
[0054]
[0055] Biphenyl bridged dinuclear iron complex D 2 The concrete steps of preparation method are:
[0056] Step 1. Ligand L 2 preparation of
[0057] Ligand L 2 The structural formula is:
[0058]
[0059] Ligand L 2 Preparation: Dissolve 10mmol benzidine and 20mmol 5-nitrosalicylaldehyde in 5mL tetrahydrofuran, stir to dissolve, add 1.0mL formic acid after the solution is clarified, and react at 80°C for 12h to obtain reaction system Ⅰ. Then the reaction system I was cooled to 25°C, a yellow solid product was precipitated, and the ligand L was obtained by suction filtration. 2 crude products. Then recrystallize with a mixed solvent of 3mL tetrahydrofuran and 6mL ethanol, and filter with suction to obtain yellow needle-like crystals. Vacuum drying at 70°C yielded 3.53 ...
Embodiment 3
[0067] A biphenyl bridged dinuclear iron complex and a preparation method thereof. The described biphenyl bridged dinuclear iron complex is denoted as D 3 , whose structural formula is:
[0068]
[0069] Biphenyl bridged dinuclear iron complex D 3 The concrete steps of preparation method are:
[0070] Step 1. Ligand L 3 preparation of
[0071] Ligand L 3 The structural formula is:
[0072]
[0073] Ligand L 3 Preparation: Dissolve 10mmol benzidine and 20mmol 5-chlorosalicylaldehyde in 4mL tetrahydrofuran, stir to dissolve, then add 0.8mL formic acid, and react at 70°C for 9h to obtain reaction system Ⅰ. Then the reaction system I was cooled to 10°C, a yellow solid product was precipitated, and the ligand L was obtained by suction filtration. 3 crude products. Then recrystallize with a mixed solvent of 2mL tetrahydrofuran and 4mL ethanol, and filter with suction to obtain yellow needle-like crystals. Vacuum drying at 70°C yielded 3.47 g of Ligand L 3 , the yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com