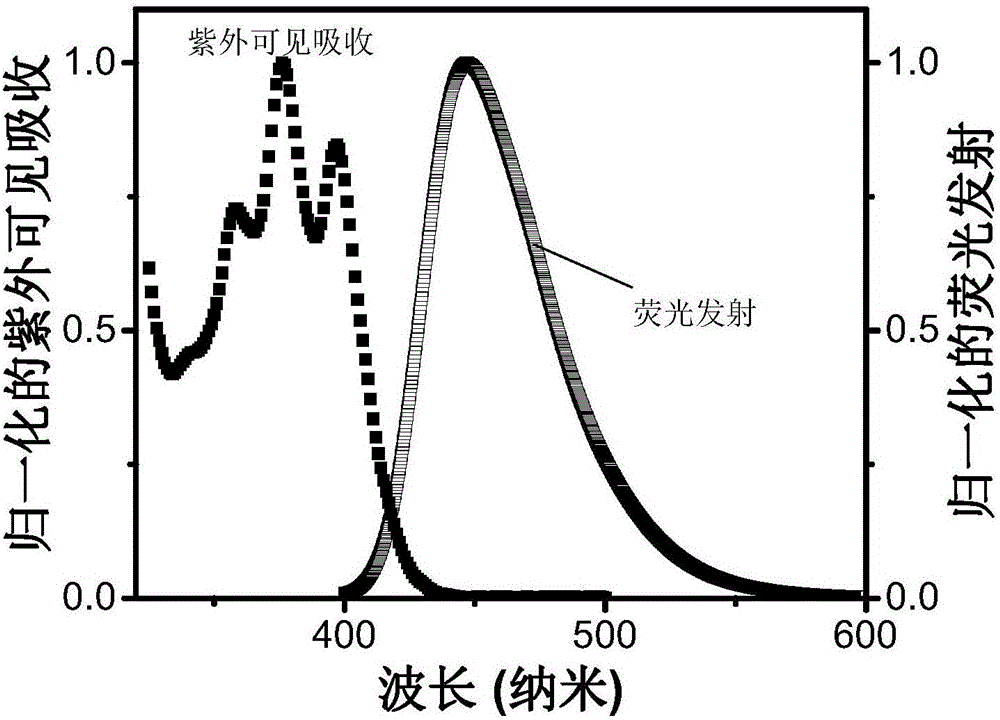
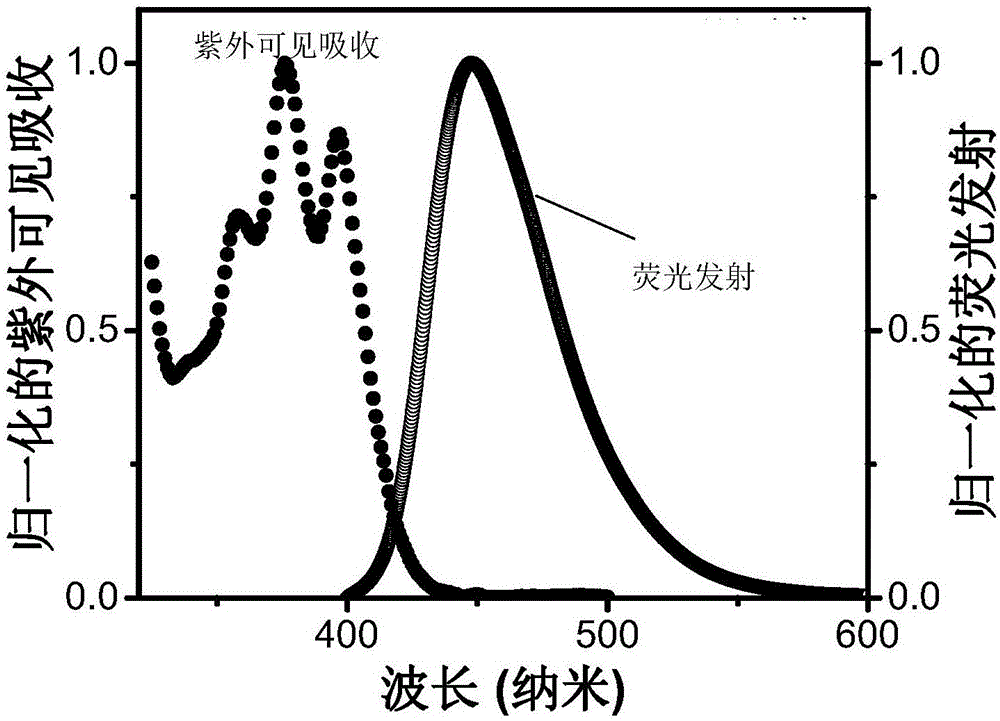
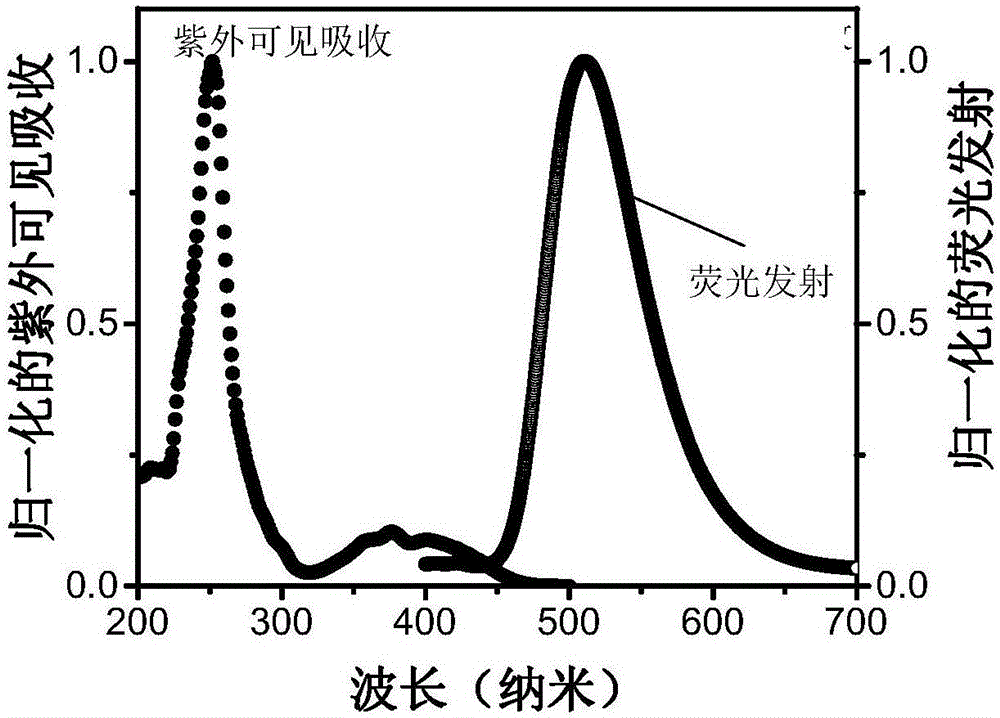
Triplet acceptor material with up-conversion circularly polarized luminescence and preparation method and application thereof
A technology of acceptor materials and luminescent materials, applied in the field of triplet acceptor materials with up-conversion circularly polarized luminescence and its preparation, which can solve the problems of failing to realize circularly polarized light emission and circularly polarized light emission, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] In this embodiment, compound R-1 is synthesized by the following method, and the synthesis process is as follows:
[0071]
[0072] The synthesis method is:
[0073] Weigh 0.28g (R)-1,1'-bi-2-naphthylamine, add it into a reaction tube containing 15mL toluene, add 30mg palladium acetate, 50mg triphenylphosphine, 200mg cesium carbonate and 0.9g 9-( 4-Bromophenyl)-10-phenylanthracene was heated to reflux under the protection of argon, and after 30 hours of reaction, the raw materials were detected by TLC to disappear, that is, the reaction was completed. After cooling down to room temperature, the reaction solution was poured into 100 mL of ethyl acetate for extraction, and the organic phase was dried with anhydrous magnesium sulfate. The solvent was spin-dried and separated by column chromatography to obtain compound R-1 with a yield of 93%.
[0074] NMR 1 H NMR (400MHz, toluene-d8) δ7.99-7.88(m, 6H), 7.85-7.64(m, 8H), 7.44(d, J=8.4Hz, 2H), 7.34-7.23(m, 8H), 7.22-7...
Embodiment 2
[0078] In this example, compound R-1 is synthesized by the following method, the synthetic process is the same as the synthetic process in Example 1, and the synthetic method is:
[0079] Weigh 0.28g of (R)-1,1'-bi-2-naphthylamine, add it into a reaction tube containing 15mL of toluene, add 45.9mg of palladium chloride, 50mg of triphenylphosphine, 318mg of cesium carbonate and 0.4g of 9 -(4-Bromophenyl)-10-phenylanthracene was heated to reflux under the protection of argon, and after 30 hours of reaction, the disappearance of the raw material was detected by TLC, that is, the reaction was completed. After cooling down to room temperature, the reaction solution was poured into 100 mL of ethyl acetate for extraction, and the organic phase was dried with anhydrous magnesium sulfate. The solvent was spin-dried and separated by column chromatography to obtain compound R-1 with a yield of 90%.
[0080] NMR 1 H NMR (400MHz, toluene-d8) δ7.99-7.88(m, 6H), 7.85-7.64(m, 8H), 7.44(d, J...
Embodiment 3
[0083]In this embodiment, compound S-1 is synthesized by the following method, and the synthesis process is as follows:
[0084]
[0085] Weigh 0.28g of (S)-1,1'-bi-2-naphthylamine, add it into a reaction tube containing 15mL of xylene, add 45mg of tetrakis(triphenyl)phosphopalladium, 180mg of sodium bicarbonate and 0.9g of 9 -(4-Bromophenyl)-10-phenylanthracene was heated to reflux under the protection of argon. After 24 hours of reaction, the disappearance of raw materials was detected by TLC, that is, the reaction was completed. After cooling down to room temperature, the reaction solution was poured into 100 mL of ethyl acetate for extraction, and the organic phase was dried with anhydrous magnesium sulfate. Spin dry solvent. Compound S-1 was separated by column chromatography with a yield of 75%.
[0086] NMR 1 H NMR (400MHz, toluene-d8) δ7.99-7.88(m, 6H), 7.85-7.64(m, 8H), 7.44(d, J=8.4Hz, 2H), 7.34-7.23(m, 8H), 7.22-7.15 (m, 6H), 7.14-6.96 (m, 12H), 6.92 (d, J=8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com