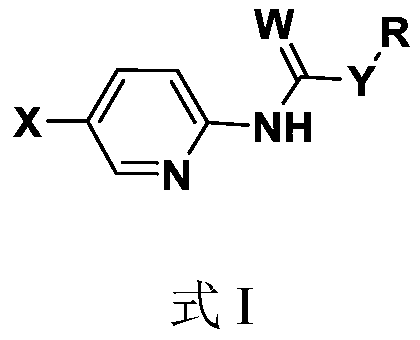
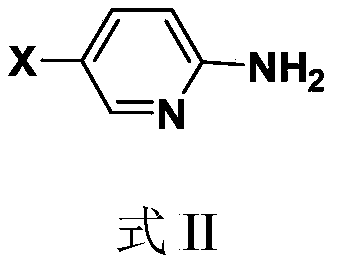
Pyridine amide compound and its preparation method and application
The technology of a compound, bromopyridine, is applied in the field of pyridine amide compounds and their preparation, which can solve the problems of brassinolide synthesis, such as high cost, many metabolic sites, and no physiological activity, so as to improve lodging resistance and facilitate preparation , good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
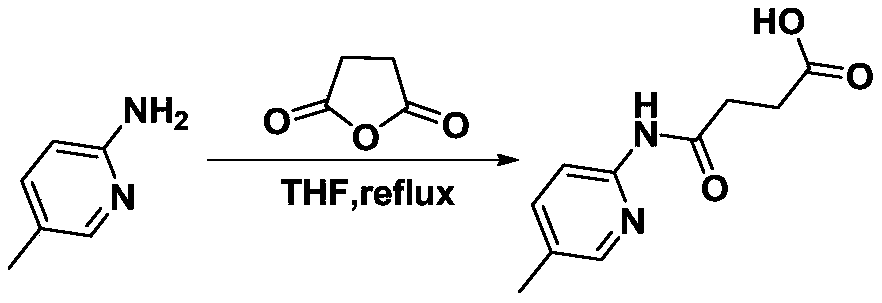
[0064] Embodiment 1, the preparation of compound 1a:
[0065] The reaction scheme is shown in the following formula:
[0066]
[0067] In a 100mL round-bottom flask, add 2-amino-5-picoline (10mmol) and succinic anhydride (12mmol), stir and dissolve 15mL tetrahydrofuran (solvent A), heat up and reflux for 2 hours for acylation reaction, cool down after the reaction A solid was precipitated, and the solid was obtained by filtration, and purified by recrystallization from ethanol. The specific method of recrystallization is: add 3 mL of ethanol dropwise to 1 g of the primary product to be recrystallized, heat to reflux until the primary product is completely dissolved, and continue to reflux for 10 minutes, then slowly cool down to precipitate a solid, and filter with suction to obtain a pure product.
Embodiment 2
[0068] Embodiment 2, the preparation of compound 1c:
[0069] The reaction scheme is shown in the following formula:
[0070]
[0071] In a 100mL round-bottomed flask, add 2-amino-5-picoline (10mmol), 3ml (20mmol) triethylamine, 15mL tetrahydrofuran and stir to dissolve, cool to 0°C in an ice-salt bath, and add the prepared solution dropwise at a constant temperature. Monoester succinyl chloride solution in 5ml tetrahydrofuran was added dropwise in 20 minutes; the ice bath was removed, the temperature was slowly raised to room temperature, and the acylation reaction continued for 2-3 hours. The reaction mixture was poured into 150mL ice water, extracted with ethyl acetate (3x 100mL), saturated Washed with brine (3 x 100 mL), dried over anhydrous Na2SO4, concentrated by filtration, and the crude product was purified by recrystallization from ethanol or ethyl acetate. The specific method of recrystallization is: add 3 mL of ethyl acetate dropwise to 1 g of the primary product ...
Embodiment 3
[0072] Embodiment 3, the preparation of compound 1g:
[0073] The reaction scheme is shown in the following formula:
[0074]
[0075] In a 100mL round bottom flask, add 2-amino-5-methylpyridine (10mmol) and 15ml tetrahydrofuran and stir to dissolve, then add 4-(trifluoromethoxy)phenylisocyanate (10mmol), and stir the reaction mixture at room temperature for acyl The reaction was carried out, and the progress of the reaction was detected by TLC. After the reaction, a large amount of solid precipitated out and was concentrated to obtain a crude product, which was purified by recrystallization from ethanol. The specific method of recrystallization is: add 3 mL of ethanol dropwise to 1 g of the primary product to be recrystallized, heat to reflux until the primary product is completely dissolved, and continue to reflux for 10 minutes, then slowly cool down to precipitate a solid, and filter with suction to obtain a pure product.
[0076] According to the same method as above...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com