8-epi-Hypophyllin E and derivatives and pharmaceutical composition thereof and application of 8-epi-Hypophyllin E in pharmacy
A technology of derivatives and compositions, applied in the field of preparation and treatment of metabolic syndrome, which can solve the problems of compound separation and no report of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Isolation, extraction and structural identification of compound 8‐epi‐hypophyllin E:
[0038] Erythema gun knife medicine (H.phyllostachya) 8kg, crushed, soaked and extracted with acetone at room temperature three times, each time for 48 hours, combined extracts concentrated under reduced pressure to obtain crude extract (950g). The crude extract was dispersed in water, extracted four times with an equal volume of ethyl acetate, and the extract was concentrated under reduced pressure. Ethyl acetate extract (680g) was subjected to silica gel column chromatography (100-200 mesh, 2kg), petroleum ether-acetone gradient elution (1:0, 8:2, 6:4, 1:1, 0:1 , the amount of each gradient solvent is 10L), which were detected by TLC and combined into five components (Fr1-Fr5). Component 2 (Fr2) was separated by reverse-phase medium-pressure liquid chromatography (MPLC, MCI), and eluted with methanol-water system gradient (70:30, 75:25, 80:20, 85:15, 90:10 and 95:5), which were det...
Embodiment 2
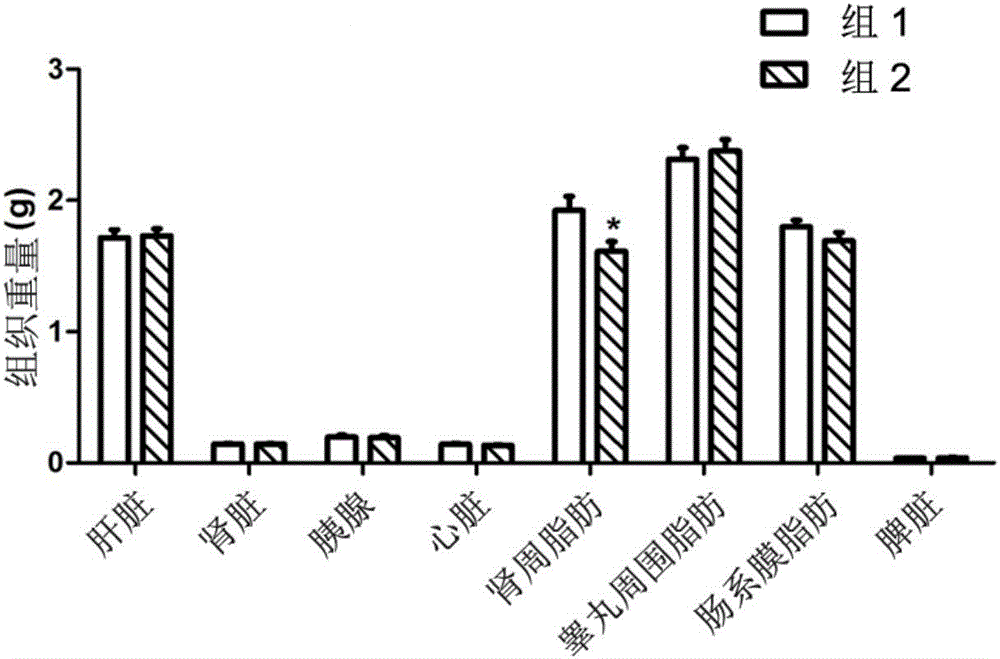
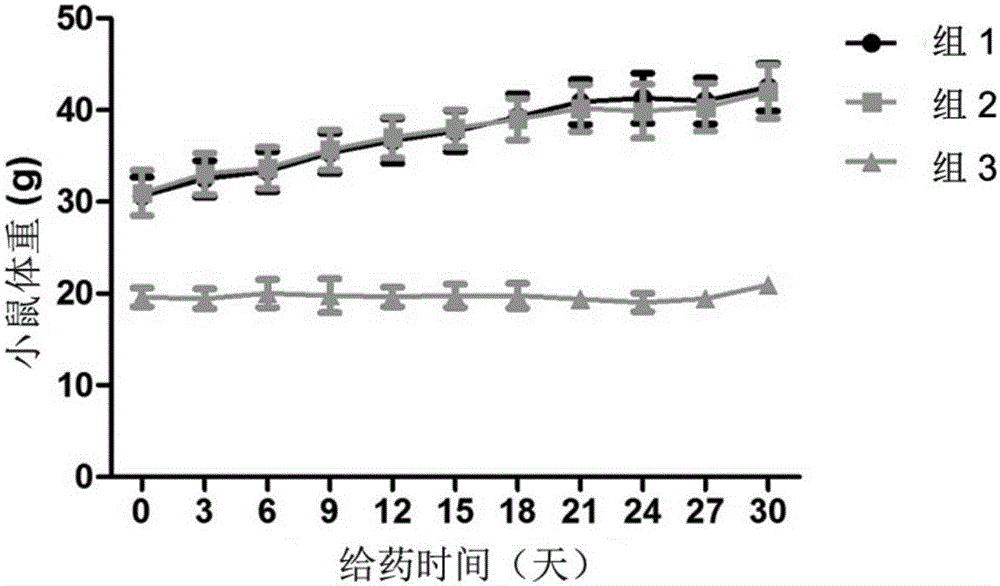
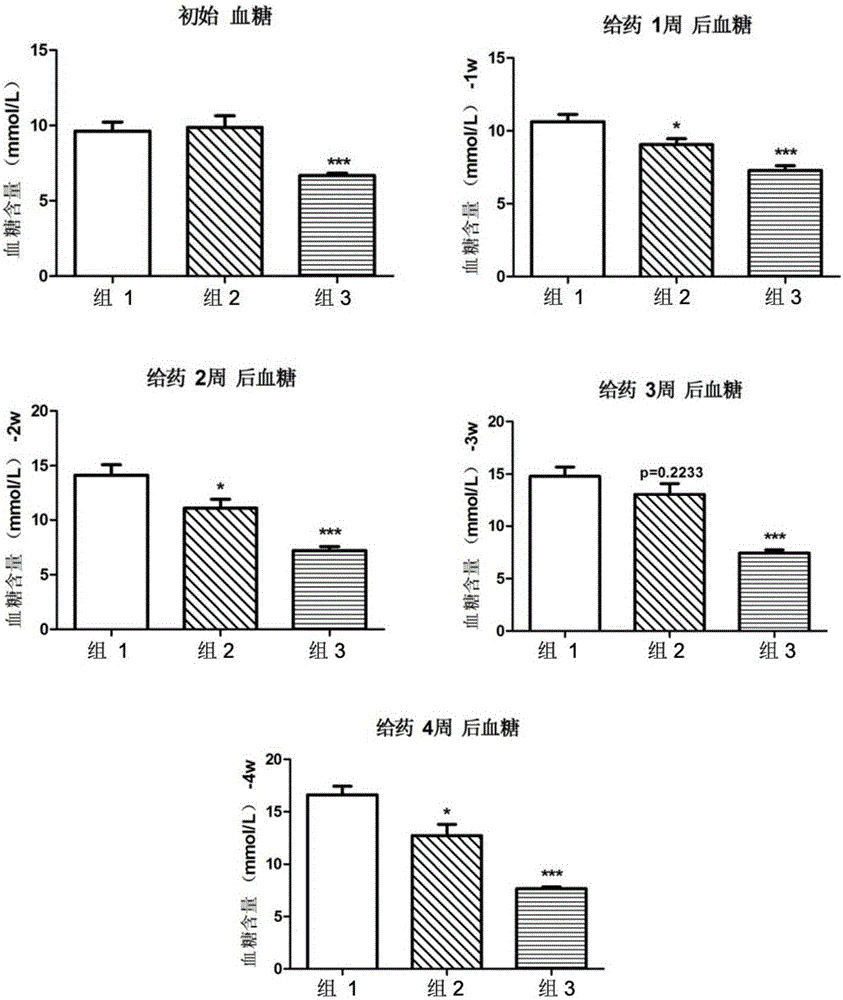
[0042] Activity of 8‐epi‐Hypophyllin E in diabetes and metabolic syndrome-related diseases.
[0043] C57BL / KsJdb / db mouse (db / db mouse) is a mouse model of type Ⅱ diabetes, mainly exhibiting leptin deficiency, hyperglycemia, hyperinsulinemia, abnormal glucose metabolism, abnormal lipid metabolism, obesity, fatty liver and other features.
[0044] The tested C57BL / KsJdb / db mice were 8-week-old female mice, SPF grade, purchased from Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The feeding conditions were raised in accordance with the SPF grade animal standard feeding operation procedures. Except for the necessary fasting time, they were allowed to eat and drink freely, and the feed was conventional feed.
[0045] Experimental Materials:
[0046] Compound 8‐epi‐hypophyllin E is a white powder, and a mixture of PEG400 and water is used as a solvent (VPEG400:Vwater=3:7). Grind it into a fine powder with a mortar first, then add PEG400 to grind until uniform...
Embodiment 3
[0097] Preparation of tablets:
[0098] The compound 8-epi-hypophyllin E was first prepared according to the method of Example 1, and the excipient was added in a weight ratio of 1:5-1:10 to the excipient, and then granulated and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com