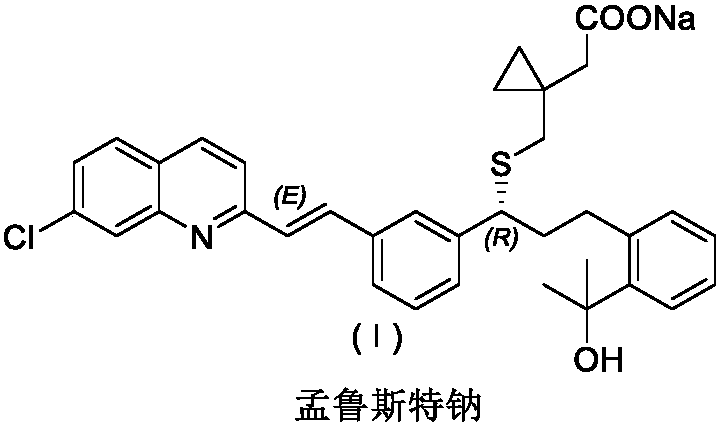
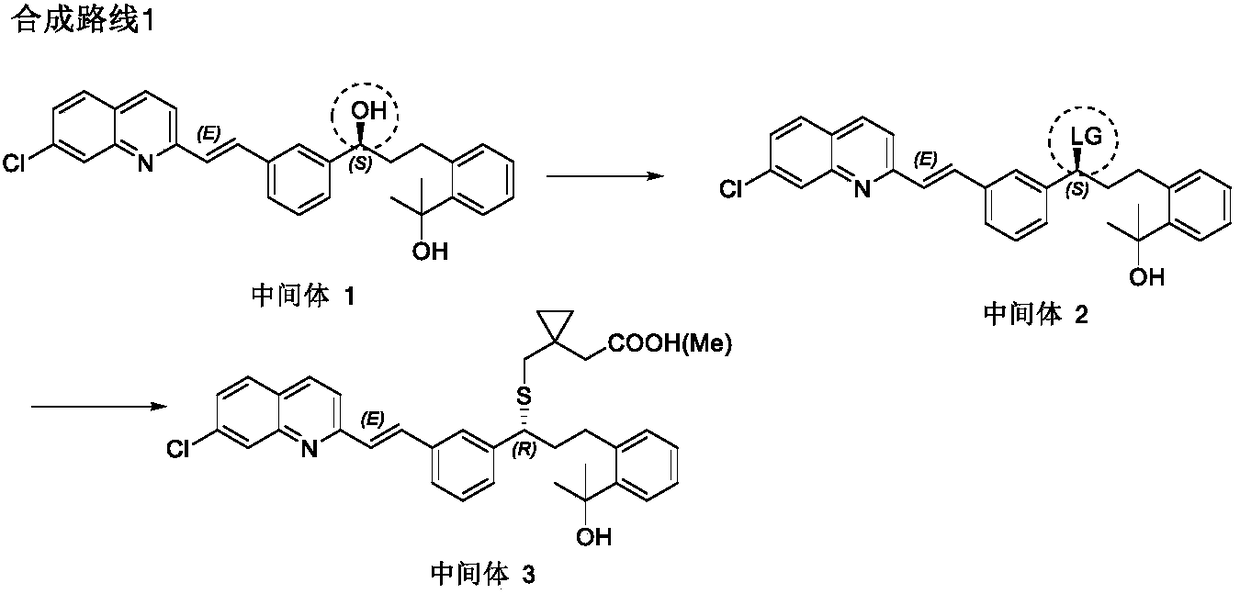
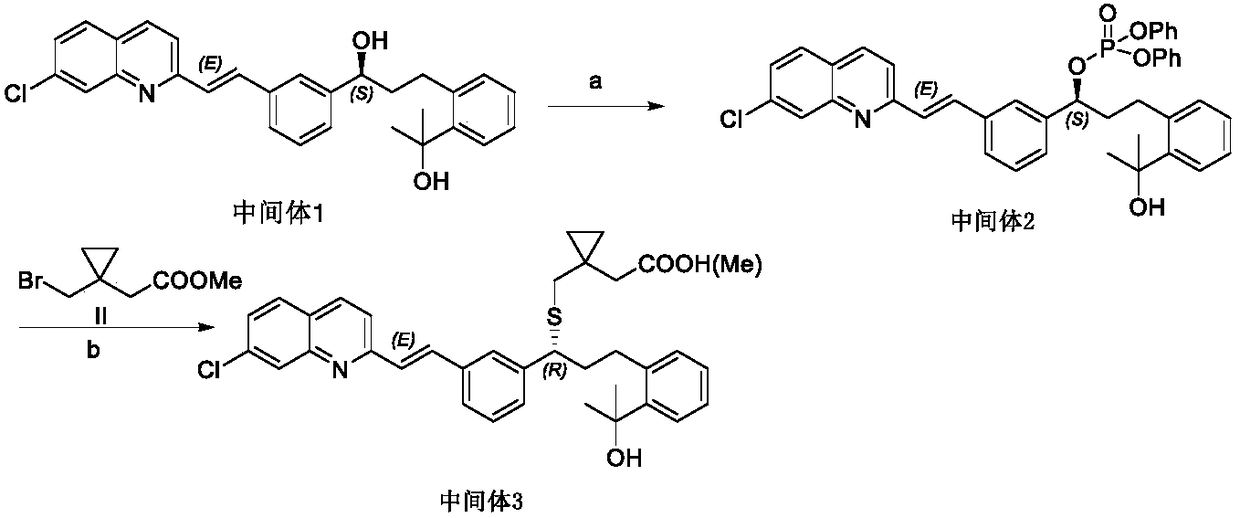
Montelukast sodium intermediate and its preparation method and application
A technology of montelukast sodium and its synthesis method, which is applied to the key intermediate of montelukast sodium and its preparation. Cyclization, easy elimination and other problems, to achieve the effect of stable chemical properties, high optical purity, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of intermediate IIIa
[0061]
[0062] The formula IVa compound (50g, 109.2mmol) was dissolved in 500mL of dichloromethane, triethylamine (22.1g, 218.4mmol) was added, and diphenyl chlorophosphate (44g, 163.8mmol) was added dropwise. Stir at 30° C. for 3 h, and TLC detects that the reaction of the raw materials is complete. Pour the reaction liquid into 500mL of 1mol / L dilute hydrochloric acid, extract and separate the organic phase, wash with saturated sodium bicarbonate solution and brine successively and collect the organic phase, dry and decolorize, then concentrate under reduced pressure to obtain 74.5g of oily formula Compound IIIa, the yield was 99%. MS:690[M+H]
[0063] 1 H-NMR (400MHz, DMSO-d 6 ),ppm:8.42-8.40(d,J=8.8Hz,1H),8.04(d,J=1.6Hz,1H),8.02-7.99(d,J=8.8Hz,1H),7.93-7.91(d, J=8.8Hz,1H),7.90-7.86(d,J=16.4Hz,1H),7.82(s,1H),7.80-7.78(d,J=7.2Hz,1H),7.75-7.73(d,J =6.8Hz,1H),7.61-7.59(dd,J=8.4Hz,1.6Hz,1H),7.51-7.50(d,J=16.4Hz...
Embodiment 2
[0064] Embodiment 2: the synthesis of intermediate IIIb
[0065]
[0066] The compound of formula IVb (50g, 105.9mmol) was dissolved in 500mL ethyl acetate, triethylamine (32.1g, 317.7mmol) was added, diphenyl chlorophosphate (56.9g, 211.8mmol) was added dropwise, after the dropwise addition was completed, Raised to 50°C and stirred for 2 h, TLC detected that the reaction of raw materials was complete. Pour the reaction solution into 500mL of 1mol / L dilute hydrochloric acid, extract and separate the organic phase, wash with saturated sodium bicarbonate solution and brine successively and collect the organic phase, dry and decolorize, then concentrate under reduced pressure to obtain 72.3g of oil The compound of formula IIIb has a yield of 97%. MS:704[M+H]
Embodiment 3
[0067] Embodiment 3: the synthesis of intermediate IIIc
[0068]
[0069] Dissolve the compound of formula IVb (30g, 63.6mmol) in 300mL of toluene, cool down to 0°C, add N,N-diisopropylethylamine (41.1g, 317.8mmol), drop diethyl chlorophosphate (54.8g , 317.8mmol), after the dropwise addition was completed, it was raised to 100° C. and stirred for 1 h, and TLC detected that the reaction of the raw materials was complete. Pour the reaction solution into 300mL of 1mol / L dilute hydrochloric acid, add 300mL of ethyl acetate for extraction, wash the organic phase with saturated sodium bicarbonate solution and brine successively and collect the organic phase, then dry, decolorize and concentrate under reduced pressure. That is, 38 g of the oily compound of formula IIIc was obtained, with a yield of 98%. MS:608[M+H]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com