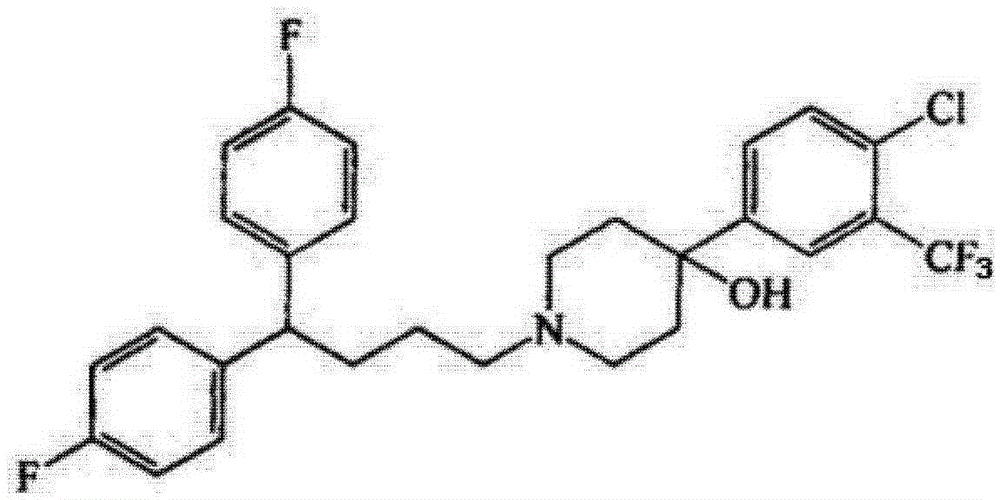
Orally disintegrating tablet containing penfluridol and preparation method of orally disintegrating tablet
A technology of orally disintegrating tablets and penfluridol, which is applied in the field of pharmaceutical preparations, can solve the problems of poor drug compliance of patients, poor drug compliance of patients, and high requirements for production equipment, and achieve improved insoluble properties, rapid onset of action, and enhanced dissolution sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Preparation Process:
[0026] (1) Penfluridol and mannitol are co-micronized in proportion;
[0027] (2) Penfluridol, mannitol, microcrystalline cellulose, and sodium carboxymethyl starch are mixed uniformly in a mixer;
[0028] (3) Add 4% hydroxypropyl cellulose aqueous solution to the above mixture to make wet soft material, and granulate;
[0029] (4) The above-mentioned granules are dried until the water content is less than 3%. After the granules are sized, the dosages of magnesium stearate, peppermint essence and aspartame are converted and mixed evenly with the dried granules;
[0030] (5) Measure the particle content after total blending, convert the tablet weight, and control the hardness to 3-5kgf for tableting. Prepared in 1000 tablets.
Embodiment 2
[0032]
[0033] Preparation Process:
[0034] (1) Penfluridol and mannitol are co-micronized in proportion;
[0035] (2) Penfluridol, mannitol, microcrystalline cellulose, and sodium carboxymethyl starch are mixed uniformly in a mixer;
[0036] (3) Add 4% hydroxypropyl cellulose aqueous solution to the above mixture to make wet soft material, and granulate;
[0037] (4) The above-mentioned granules are dried until the water content is less than 3%. After the granules are sized, the dosages of magnesium stearate, peppermint essence and aspartame are converted and mixed evenly with the dried granules;
[0038] (5) Measure the particle content after total blending, convert the tablet weight, and control the hardness to 3-5kgf for tableting. Prepared in 1000 tablets.
Embodiment 3
[0040]
[0041] Preparation Process:
[0042] (1) Penfluridol and mannitol are co-micronized in proportion;
[0043] (2) Penfluridol, mannitol, microcrystalline cellulose, and sodium carboxymethyl starch are mixed uniformly in a mixer;
[0044] (3) Add 4% hydroxypropyl cellulose aqueous solution to the above mixture to make wet soft material, and granulate;
[0045] (4) The above-mentioned granules are dried until the water content is less than 3%. After the granules are sized, the dosages of magnesium stearate, peppermint essence and aspartame are converted and mixed evenly with the dried granules;
[0046] (5) Measure the particle content after total blending, convert the tablet weight, and control the hardness to 3-5kgf for tableting. Prepared in 1000 tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com