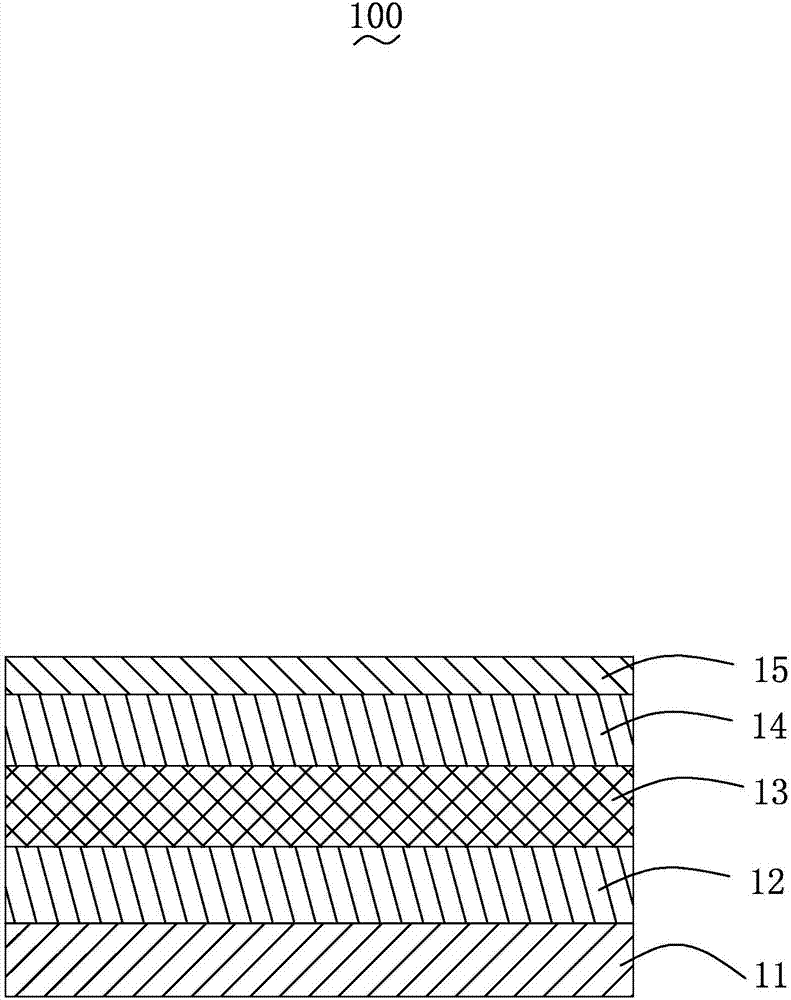
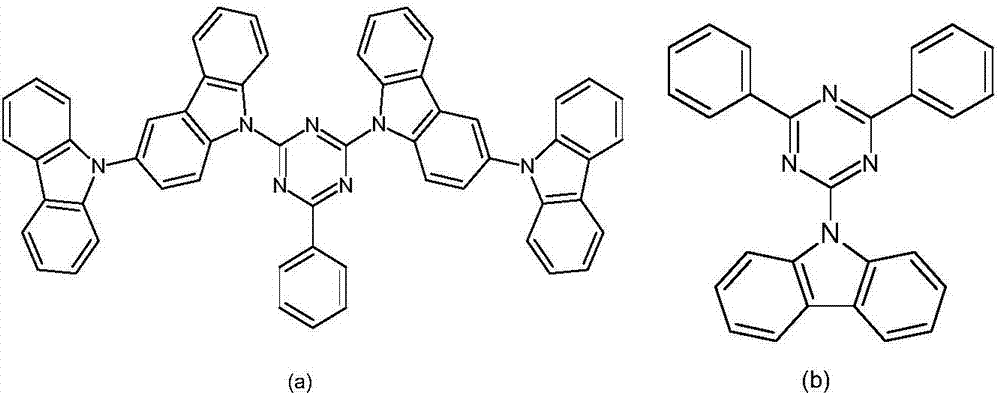
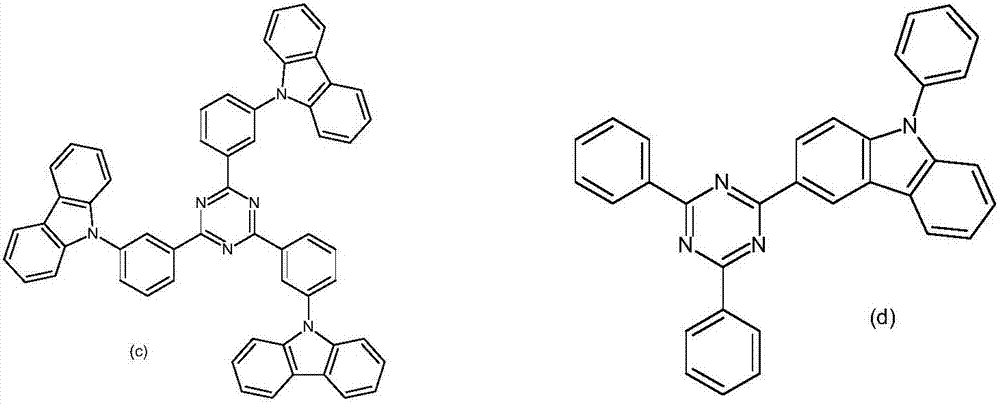
Triazine compound and luminescent device
A technology of triazine compounds and compounds, which is applied in the field of triazine compounds and light-emitting devices, can solve the problems of unfavorable charge balance, carrier transport, and large hole transport performance, and achieve low triplet and singlet energy level difference, Effect of improving luminous efficiency and prolonging device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Synthesis of compound TA-1-1
[0122]
[0123] Dissolve 2mmol of the raw material (S1-2) in 25mL of tetrahydrofuran, add dropwise a butyllithium solution containing 2mmol of butyllithium under nitrogen protection, and stir for 10 minutes, then add the solvent dropwise to contain 1mmol of the raw material (S1-1) In 25mL tetrahydrofuran solution. After stirring for 10 minutes, the temperature was raised to reflux for 6 hours. A solid was precipitated by adding 100 mL of water, and the solid was washed with water and n-hexane in turn. Recrystallization from ethanol yielded intermediate (S1-3). Take 1mmol of intermediate (S1-3), 1.2mmol of aromatic boronic acid compound (S1-4), 0.1g of tetrakis(triphenylphosphine palladium), 3mL of 2mol / L sodium carbonate solution, 4mL of toluene and 2mL of ethanol, nitrogen Heat and stir at 80°C for 8 hours under protective conditions. The reaction solution was extracted with dichloromethane, washed with water, dried with anhydrous sodium...
Embodiment 2
[0125] Synthesis of compound TA-1-2
[0126] The same operation method as in Synthesis Example 1 was adopted except that the aromatic boronic acid compound (S1-4) was replaced with (S2-4) to obtain compound TA-1-2. The detection yield is 36%, the product mass spectrum (m / e): 640.1, ΔE ST =0.29eV.
[0127]
Embodiment 3
[0129] Synthesis of compound TA-1-3
[0130] The same operation method as in Synthesis Example 1 was adopted except that the aromatic boronic acid compound (S1-4) was replaced with (S3-4) to obtain the target compound TA-1-3. The detection yield is 39%, the product mass spectrum (m / e): 640.2, ΔE ST = 0.28eV.
[0131]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com