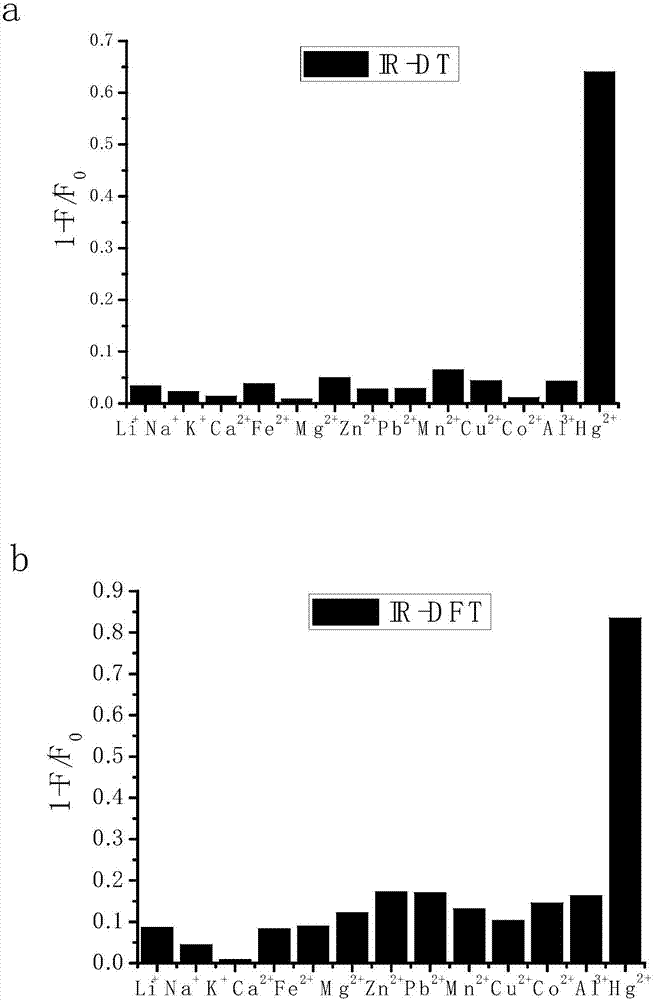
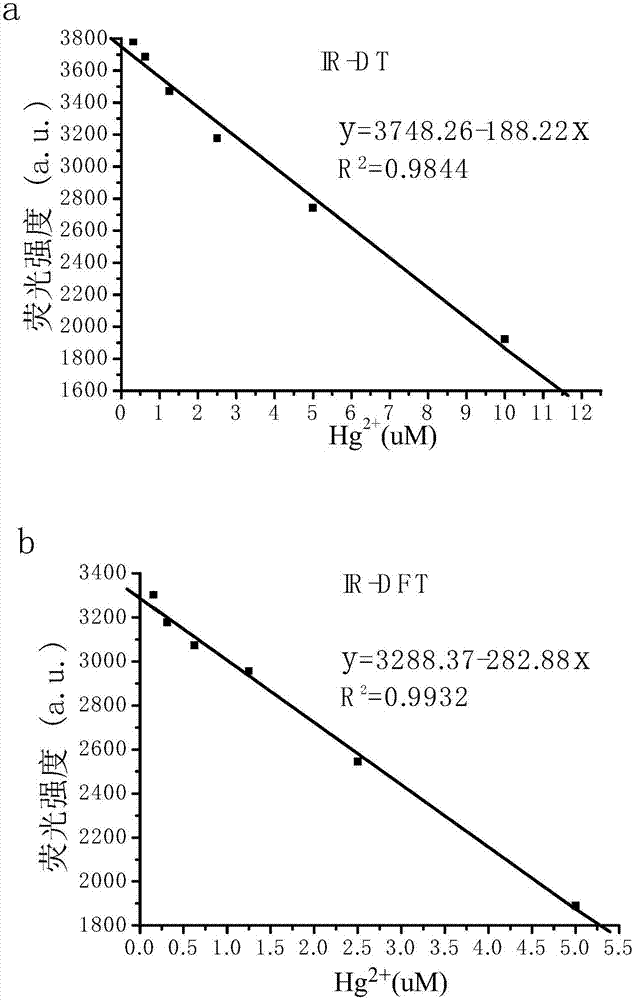
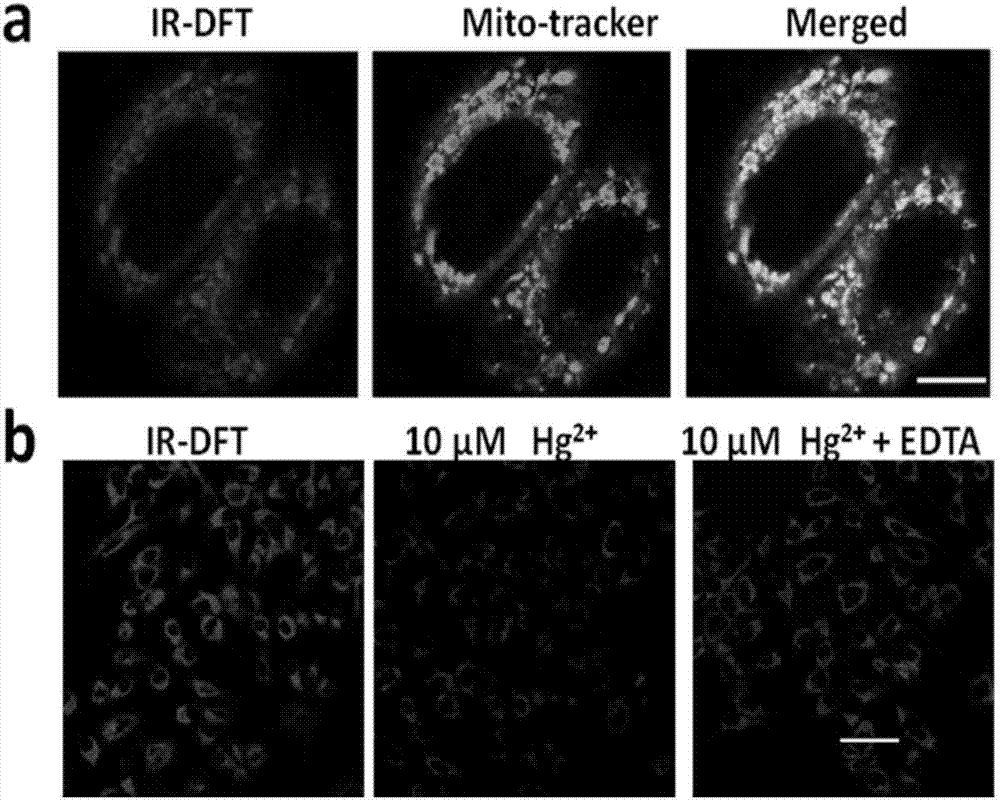
Uridylated heptamethine indocyanine dye as well as preparation method and application
A kind of technology of indole heptamethanine and uracil, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 dialdehyde chlorocyclohexene (2-chloro-3-hydroxymethane-1-formyl cyclohexene)
[0040]
[0041] In the reactor containing 60ml of anhydrous DMF, slowly drop 20ml of POCl under stirring 3 , and control the internal temperature not higher than 5°C. Add 5ml of cyclohexanone after dropping, and control the internal temperature not to exceed 10°C. After dropping, the oil was heated to raise the internal temperature to 80°C, and the reaction was stirred for 3.5 hours. Stop the reaction, pour the reaction solution into water, and stir for 3 hours. A large number of yellow solids precipitated, filtered with suction, and the filter cake was washed with cold water until neutral. The crude product was refined with ethyl acetate to obtain 5.2 g of yellow granular crystals, with a yield of 65.3%. Store away from light and low temperature (4°C). Melting point: 130-131°C (literature melting point: 130-131°C) TLC detection: cyclohexane: ethyl acet...
Embodiment 2
[0043] Example 2 Preparation of 1-(3-bromopropyl)pyrimidine-2,4(1H,3H)-dione (Compound 1)
[0044]
[0045] Add 12.0 mmol of uracil or fluorouracil to a 50 mL reaction flask, add hexamethyldisilamine (HMDS, 48.0 mmol) and 0.25 mL of trimethylchlorosilane under nitrogen protection, and then heat and stir at 126°C for reaction. After 5h, directly concentrate under reduced pressure to remove the solvent. The residue was added with 10mL of 1,3-dibromopropane, heated and stirred at 105°C to react. After 5h, it was directly concentrated under reduced pressure, the residue was dissolved in dichloromethane, washed with water, washed with saturated brine, separated, the organic layer was dried over anhydrous sodium sulfate, filtered with suction, concentrated, and purified by column separation (elution condition: CH 2 Cl 2 / CH 3 OH=50 / 1) to obtain compound 1a or 1b.
[0046] 1a (82%): 1 H NMR (400MHz, CDCl 3 ):δ11.352(s,1H),7.47-7.45(d,J=8Hz,1H),7.27-7.26(d,J=8Hz,1H),7.04-6.98...
Embodiment 3
[0048] The preparation of embodiment 3N-thymine indole quaternary ammonium bromide salt (2a, 2b)
[0049]
[0050] Take a 25mL reaction flask (with condensing reflux device), add compound 1a or 1b (1.83mmol), indole (3.66mmol). Under nitrogen protection, anhydrous acetonitrile (6 mL) was added to dissolve, and the reaction was heated and stirred at 81°C. After 22 hours, the reaction was stopped, and after the reaction solution was cooled to room temperature, the pressure was reduced directly to obtain a dark red viscous substance. The viscous material was washed with isopropyl ether and dried to obtain the crude product of the target compound (2a, 2b). The next reaction was carried out without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com