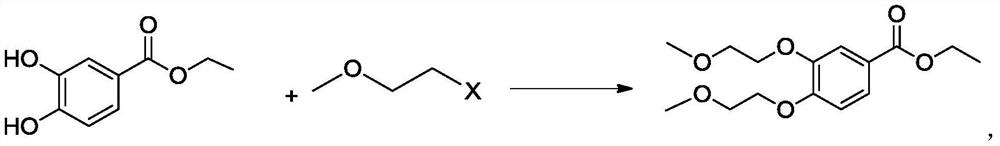
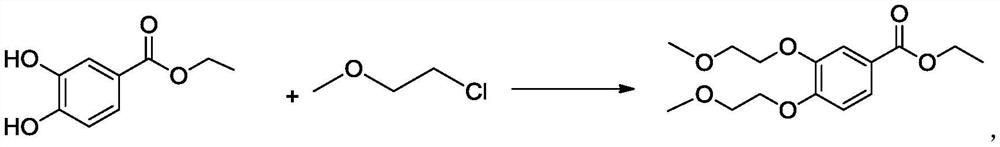
A kind of preparation method of 3,4-two (2-methoxyethoxy) ethyl benzoate
A technology of ethyl dihydroxybenzoate and methoxyethoxy, which is applied in the field of drug synthesis and can solve problems such as not being environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 36.4g ethyl 3,4-dihydroxybenzoate, 82.4g potassium carbonate, 6.0g 4-dimethylaminopyridine, 145.6g 1-chloro-2-methoxyethane into a dry and clean 500ml reaction bottle and stir Raise the temperature to 90-95°C, reflux and keep it warm, after the reaction is completed, cool down to 20-30°C, add water and stir, let stand to separate layers, extract the water layer once, combine the organic layers, distill and evaporate, and cool down. Add isopropanol and water, filter with suction, rinse the filter cake with isopropanol and water, drain the wet product, and dry it in vacuum to obtain 57g of the product, with a yield of 95% and a purity of 99% by HPLC.
Embodiment 2
[0032] Add 36.4g ethyl 3,4-dihydroxybenzoate, 82.4g potassium carbonate, 4.0g 4-dimethylaminopyridine, 145.6g 1-chloro-2-methoxyethane into a dry and clean 500ml reaction bottle and stir Raise the temperature to 85-90°C, reflux and keep it warm, after the reaction is completed, cool down to 20-30°C, add water and stir, let stand to separate layers, extract the water layer once, combine the organic layers, distill and steam, and cool down. Add isopropanol and water, filter with suction, rinse the filter cake with isopropanol and water, drain the wet product, and dry it in vacuum to obtain 57g of the product, with a yield of 95% and a purity of 99% by HPLC.
Embodiment 3
[0034] Put 36.4g of ethyl 3,4-dihydroxybenzoate, 82.4g of potassium carbonate, 145.6g of 1-chloro-2-methoxyethane into a dry and clean 500ml reaction bottle, stir and raise the temperature to 85-90°C, and keep the temperature under reflux After the reaction is completed, cool down to 20-30°C, add water and stir, let stand to separate layers, extract the water layer once, combine the organic layers, distill, evaporate, and cool down. Add isopropanol and water, filter with suction, rinse the filter cake with isopropanol and water, drain the wet product, and dry it in vacuum to obtain 54 g of the product, with a yield of 90% and a purity of 97% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com