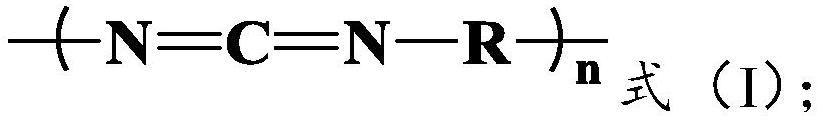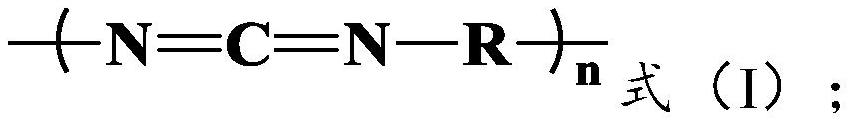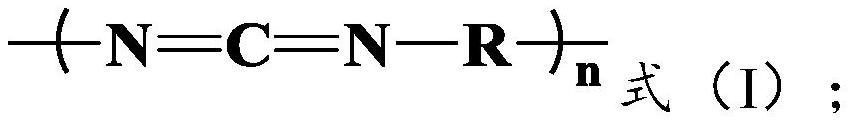A kind of method for preparing lactide from lactic acid oligomer
A technology of lactic acid oligomer and lactide, applied in the direction of organic chemistry and the like, can solve the problems of too high production, unfavorable production, long dehydration polycondensation time, etc., and achieves the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In the three-necked flask (5000 mL) with a magnetic stirrer and a thermometer, add 3.0 kg of L-lactic acid (the content of lactic acid is 93%), and add stannous benzoate according to 0.3 wt % of the amount of lactic acid. Dehydrate under the stirring condition of vacuum degree of -0.095~-0.1MPa, slowly raise the temperature to 160℃~170℃, and then continue dehydration at 160~170℃ for 10h to obtain lactic acid oligomers, and measure the number average of lactic acid oligomers Molecular weight (M n ) is 3984 g / mol.
Embodiment 2
[0046] The lactic acid oligomer 140g of embodiment 1 is dissolved with an appropriate amount of dichloromethane, joins in pre-dried three-necked bottle with magnetic stirrer and thermometer, and adds active compound isophorone diisocyanate (IPDI) The molar ratio of the isocyanate group (-NCO) to the oligomer is 1.90:1, and the reaction is stirred at room temperature for 8h, then most of the dichloromethane is distilled off, and the remaining dichloromethane is extracted by vacuum, heated to Keep at 180°C for 10 minutes, and crack the lactic acid oligomers under the condition of the temperature of 190°C to 210°C and the vacuum degree of -0.095 to -0.1MPa until there is no more lactide distilled out in the three-necked bottle. The lactide produced by the cracking is condensed in the tube and collected.
[0047] The obtained lactide was detected, and the content of free acid was 88ppm, and the yield of lactide was 87.4%.
Embodiment 3
[0049] According to Example 2, the difference is that the addition of active compound IPDI is that the molar ratio of isocyanate group (-NCO) to oligomer is 2.46:1, and the content of free acid in the obtained lactide is 79ppm, and the production of lactide The rate is 85.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com