Fluorinated perylene diimide derivative and application thereof
A technology of fluorinated peryleneimide and substituted peryleneimide, which is applied in the field of fluorinated peryleneimide derivatives and its application in organic solar cells, and can solve the problem of long energy repayment time and inappropriate Preparation of flexible devices and other issues to achieve the effect of increasing charge mobility, reducing recombination energy, and large electron affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
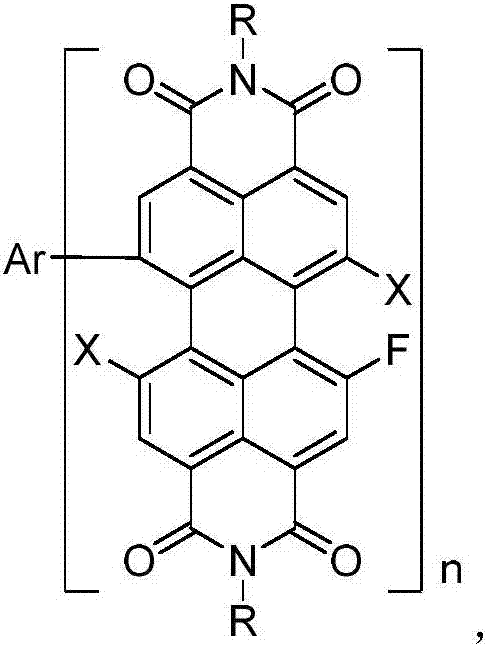
[0017] The preparation process of fluorinated perylene imide derivatives of the present invention is as follows:
[0018] (a) under protective atmosphere, join the compound with formula (I) structure and cesium fluoride in dioxane, then add octadecyl-crown ether-6 and reflux overnight reaction (the moles of corresponding three kinds of reaction raw materials Ratio 1:20-40:1-2), after washing, extracting, drying and column chromatography, the bisfluoroperyleneimide having the structure of formula (II) is obtained,
[0019]
[0020] (b) Under a protective atmosphere, add bisfluoroperyleneimide having the structure of formula (II) and lithium bromide (molar ratio 1:1) into 1-methyl-2-pyrrolidone, at 200-250°C After reacting for 4-8h, the reaction was quenched with water, the organic phase was combined after washing and extraction and spin-dried, and finally the compound with the structure of formula (III) was obtained after separation by column chromatography,
[0021]
[...
Embodiment 1
[0026] Under the protective atmosphere of argon, N,N'-diisooctyl-1,7-dibromo-perylene diimide (1g, 1.83mmol, 1eq) and cesium fluoride (8.34g, 54.93mmol, 30eq ) was added to 15ml of dioxane, then 18-crown-6 (968mg, 3.66mmol, 2eq) was added, and the reaction was refluxed overnight. After the product was washed with water, it was extracted three times with dichloromethane. After drying, the solvent was removed by rotary evaporation, and silica gel column chromatography was performed with dichloromethane as the eluent to obtain N,N'-diisooctyl-1,7-difluoro-perylenediimide.
[0027] Under an argon atmosphere, N,N'-diisooctyl-1,7-difluoro-perylene diimide (1g, 2.36mmol, 1eq) and lithium bromide (205mg, 2.36mmol, 1eq) were added to In 20ml of 1-methyl-2-pyrrolidone, react at 220°C for 5h, then quench the reaction with water, wash the reactant with water, then extract three times with chloroform, combine the organic phases and dry them with anhydrous sodium sulfate, and remove the sol...
Embodiment 2
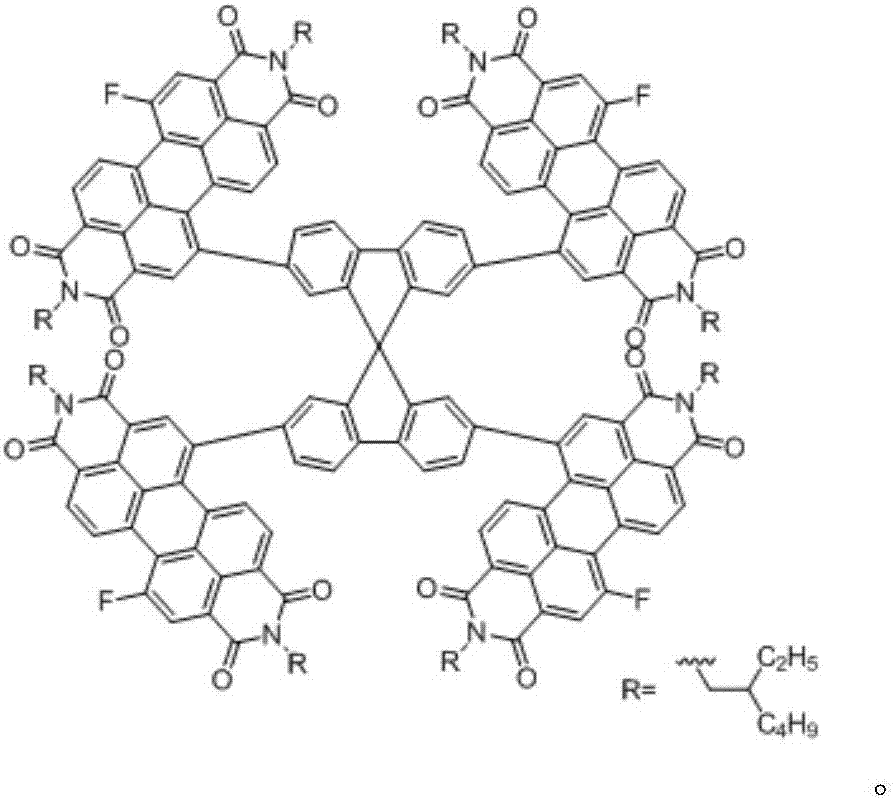
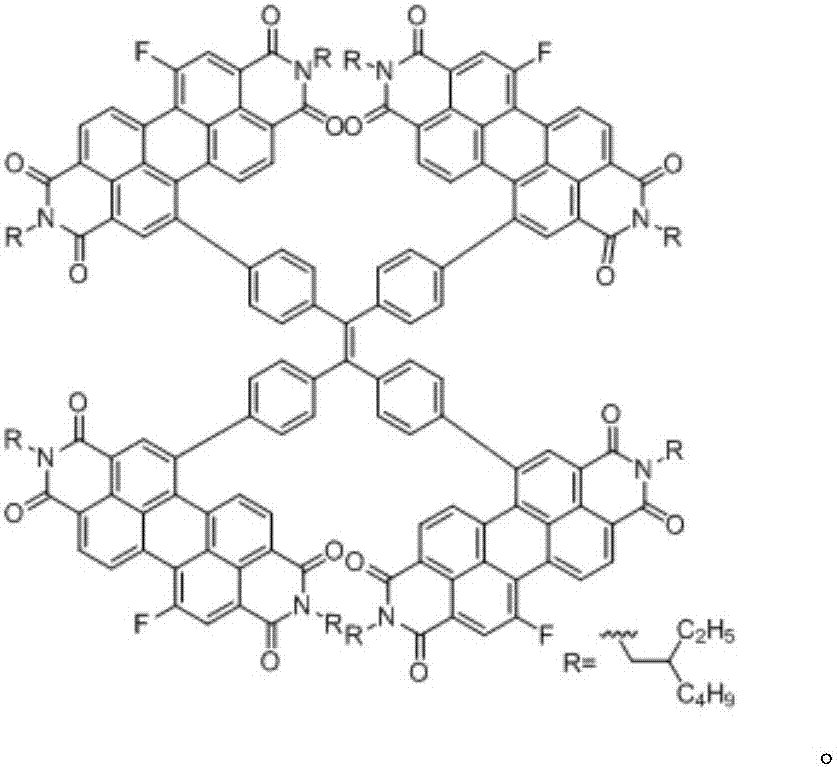
[0031] Under the protective atmosphere of argon, tetraphenylethylene units (1g, 1.2mmol, 1eq) connected with four borate esters and N,N'-diisooctyl-1-bromo-7-fluoro-perylenediyl Imine (4.25g, 5.98mmol, 5eq) was added to tetrahydrofuran / water (20ml / 5ml) mixed solvent, and then Pd(PPh 3 ) 4 (53.4mg, 0.060mmol, 0.05eq) and potassium carbonate (1.65g, 12.0mmol, 10eq), reacted at 80°C for 2 days, washed the reactant with water after the reaction, extracted three times with dichloromethane, combined the organic phases, no After the organic phase was dried with sodium sulfate, the solvent was removed by rotary evaporation, and silica gel column chromatography was performed with dichloromethane as eluent to obtain the target compound 5b.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com