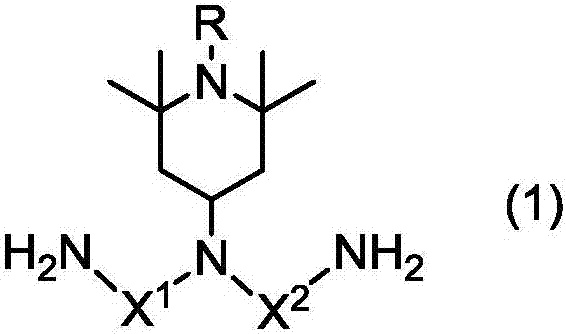
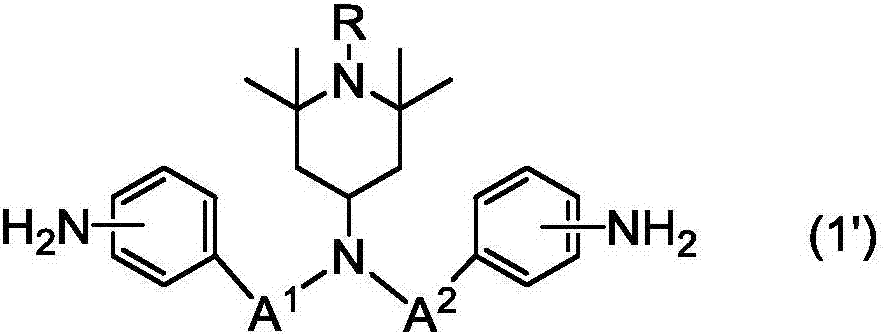
Gia Min, Polya Mick acid or its derivative, a rubbing agent, a film for liquid crystal display , and a liquid crystal display element
A technology of polyamic acid and derivatives, used in liquid crystal materials, chemical instruments and methods, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
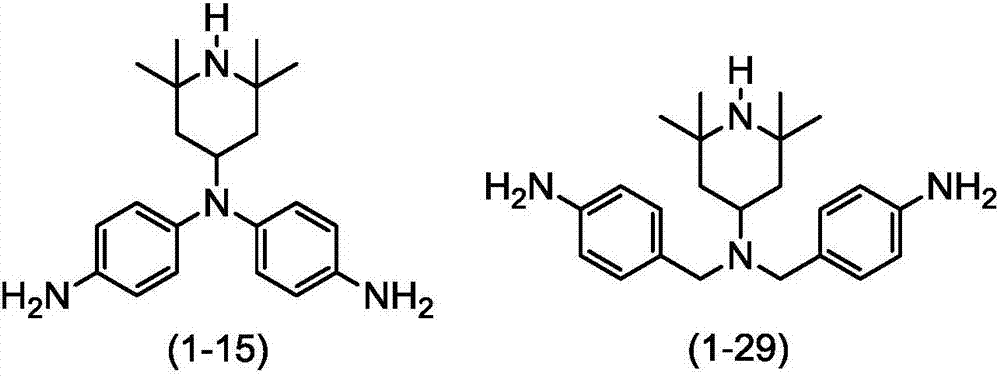
[0758] Synthesis of diamine represented by formula (1-15).
[0759] Synthesis of Dinitroform
[0760] Put 4-amino-2,2,6,6-tetramethylpiperidine (20.0g, 128.0mmol) and potassium carbonate (44.2g, 320.0mmol) into a 500mL three-necked flask equipped with a thermometer and a reflux tube, add 200mL DMF. The solution was kept below 5°C, and 4-fluoronitrobenzene (39.7 g, 281.6 mmol) was slowly added thereto. The cooling of the reaction solution was stopped, and the mixture was further stirred at room temperature for 24 hours. The reaction solution was poured into 500 mL of pure water, and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, and the desiccant was filtered off, and the solvent was distilled off under reduced pressure to obtain a crude product. The obtained crude product was recrystallized from ethanol (50 mL) to obtain the following compound. Yield was 26.3 g, yield 52%.
[0761]
[0762] Reduction of Nitro
[0763] The c...
Embodiment 2
[0765] The synthesis of the diamine shown in formula (1-29)
[0766] Synthesis of Dinitroform
[0767] Put 4-amino-2,2,6,6-tetramethylpiperidine (20.0g, 128.0mmol) and potassium carbonate (44.2g, 320.0mmol) into a 500mL three-necked flask equipped with a thermometer and a reflux tube, add 200mL DMF. The solution was kept below 5° C., and a solution obtained by dissolving 4-nitrobenzyl bromide (60.8 g, 281.6 mmol) in DMF (50 mL) was slowly added thereto. The cooling of the reaction solution was stopped, and the mixture was further stirred at room temperature for 5 hours. The reaction solution was poured into 500 mL of pure water, and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, and the desiccant was filtered off, and the solvent was distilled off under reduced pressure to obtain a crude product. The obtained crude product was recrystallized from ethanol (50 mL) to obtain the following compound. Yield was 46.2 g, 85% yield.
[0...
Embodiment 3
[0772] The synthesis of the diamine shown in formula (1-1)
[0773] Mesylation
[0774] 2-Nitroethanol (30.0 g, 329.4 mmol) and triethylamine (50.0 g, 494.2 mmol) were charged into a 2000 mL three-necked flask equipped with a thermometer and a reflux tube, and 600 mL of THF was added. The solution was kept below 5°C, and methanesulfonyl chloride (45.3 g, 395.3 mmol) was slowly added thereto. The cooling of the reaction solution was stopped, and the mixture was further stirred at room temperature for 24 hours. The reaction solution was poured into 500 mL of pure water, and extracted with ethyl acetate. The organic layer was dried over sodium sulfate anhydrate, and the desiccant was filtered off, and the solvent was distilled off under reduced pressure to obtain the following compound. The yield was 53.2 g, the yield was 95%.
[0775]
[0776] Synthesis of Dinitroform
[0777] Put 4-amino-2,2,6,6-tetramethylpiperidine (13.5g, 86.5mmol) and potassium carbonate (27.5g, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ion density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com