Method for recovering valuable metals by comprehensively treating high-iron and low-germanium zinc oxide calcine and zinc sulfite
A technology of zinc sulfite and valuable metals, applied in the field of hydrometallurgy, can solve the problems of lowering the grade of germanium concentrate, injury to operators, formation of enrichment and failure to open the circuit, etc., to solve the difficulty of liquid-solid separation and save resources , the effect of avoiding poisoning risk and environmental protection risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]The high-iron and low-germanium zinc oxide calcine produced by Yunnan Luoping Zinc Electric Co., Ltd. hydrometallurgy multi-hearth furnace and the zinc sulfite produced by the sulfur dioxide tail gas absorption workshop of the rotary kiln were used. After drying, 20000.00 grams of samples were shrunk and divided for use. The main components of its high iron and low germanium zinc oxide calcine are: zinc 35.06wt%, germanium 0.011wt%, lead 8.14wt%, fluorine 0.010wt%, chlorine 0.009wt%, iron 6.21wt%, silver 0.006wt%; The main components of zinc are: zinc 30.09wt%, germanium 0.012wt%, lead 5.98wt%, fluorine 0.0059wt%, chlorine 0.0081wt%, iron 6.88wt%, silver 0.007wt%.
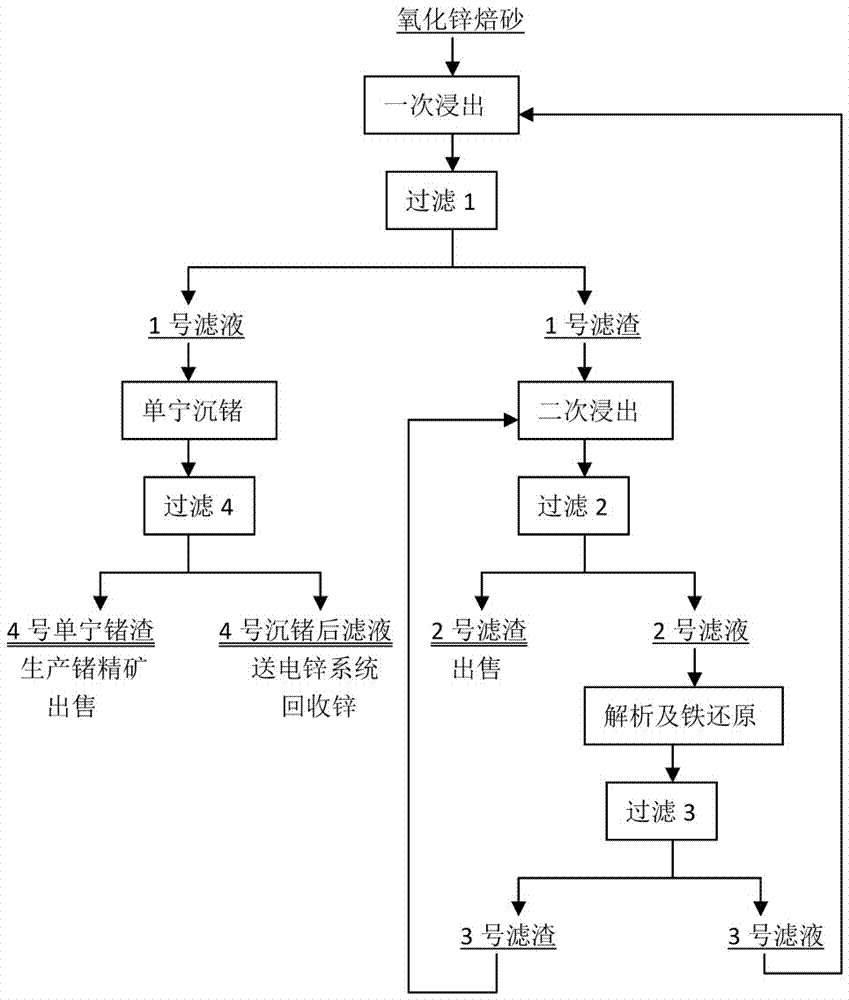
[0033] (1) Primary leaching: use industrial water as pre-liquid, take 75 liters, add zinc oxide calcined sand to make slurry for 30 minutes, adjust the initial acid to 130g / l with concentrated sulfuric acid, and control the process temperature to 80 ℃, leaching time 1.5 hours, end point pH = 3.0, then filter ...
Embodiment 2
[0043] The high-iron and low-germanium zinc oxide calcine produced by Yunnan Luoping Zinc Electric Co., Ltd. hydrometallurgy multi-hearth furnace and the zinc sulfite produced by the sulfur dioxide tail gas absorption workshop of the rotary kiln were used. After drying, 20000.00 grams of samples were shrunk and divided for use. The main components of the high iron and low germanium zinc oxide calcine are: zinc 44.96wt%, germanium 0.022wt%, lead 11.96wt%, fluorine 0.015wt%, chlorine 0.020wt%, iron 8.04wt%, silver 0.008wt%. The main components of the zinc sulfite are: zinc 39.83wt%, germanium 0.019wt%, lead 10.06wt%, fluorine 0.099wt%, chlorine 0.0098wt%, iron 8.97wt%, silver 0.009wt%.
[0044] (1) One-time leaching: use industrial water as pre-liquid, take 75 liters, add zinc oxide calcine to 10700.0 grams for slurrying, slurrying time is 40 minutes, use concentrated sulfuric acid to adjust the initial acid to 140g / l, control the process temperature 85 ℃, the leaching time is 2...
Embodiment 3
[0054] The high-iron and low-germanium zinc oxide calcine produced by Yunnan Luoping Zinc Electric Co., Ltd. hydrometallurgy multi-hearth furnace and the zinc sulfite produced by the sulfur dioxide tail gas absorption workshop of the rotary kiln were dried and shrunk for sample preparation. The high iron and low The main components of germanium zinc oxide calcine are: zinc 35wt%, germanium 0.01wt%, lead 8wt%, fluorine 0.01wt%, chlorine 0.01wt%, iron 6wt%, silver 0.006wt%. The main components of the zinc sulfite are: zinc 30wt%, germanium 0.01wt%, lead 6wt%, fluorine 0.006wt%, chlorine 0.008wt%, iron 7wt%, silver 0.007wt%.
[0055] 1) Primary leaching: Slurry the zinc oxide calcined sand with a liquid-solid ratio of 6:1 with a slurrying solution, the slurrying time is 30 minutes, adjust the initial acid 130g / l with concentrated sulfuric acid, control the process temperature at 80°C, and the leaching time After 1.5 hours and the end point pH=3.0, filter 1, filter 1 to produce No...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com