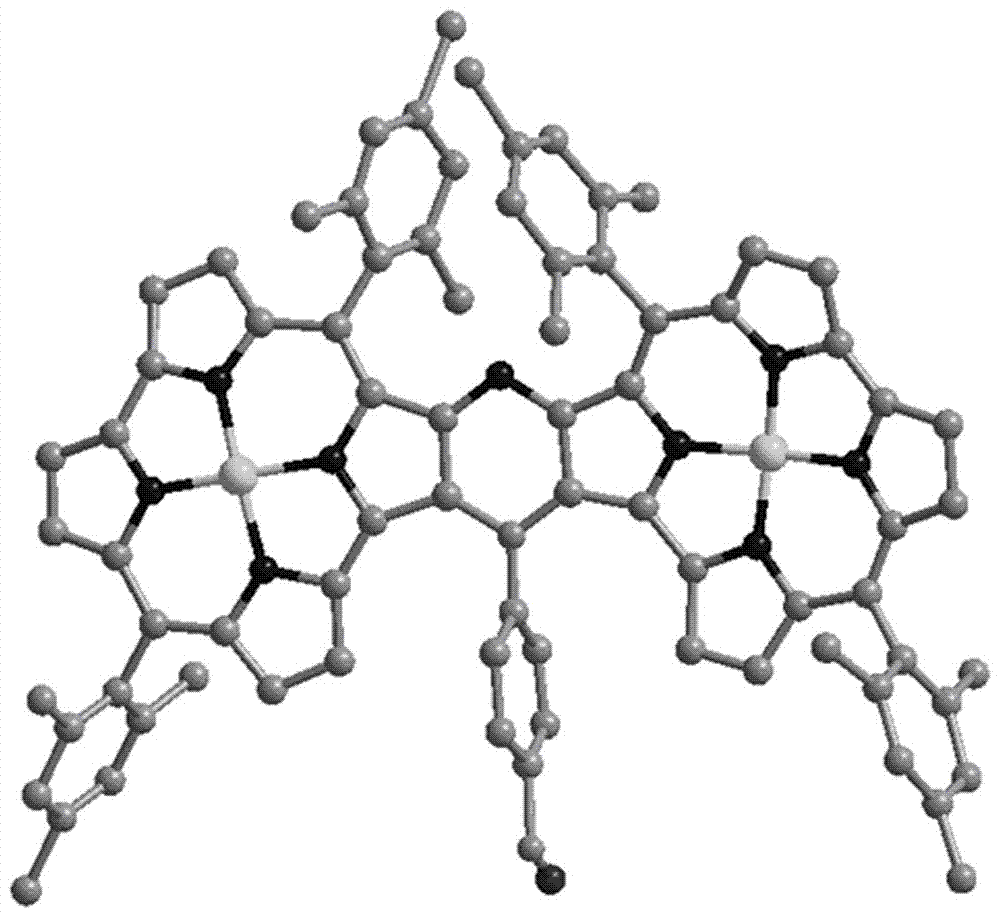
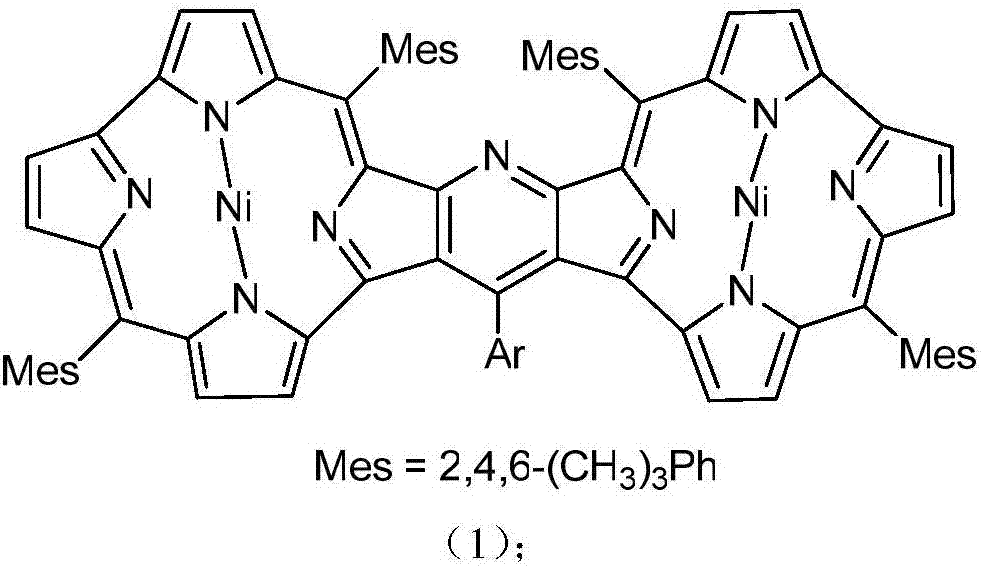
Pyridine fused norcorrole compound and preparation method thereof
A technique for pyridine fusion and compound, which is applied in the field of pyridine fusion norcarbrole compound and its preparation, can solve the problems that have not been reported in the literature, and achieve the effects of good reaction selectivity, high yield and stable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
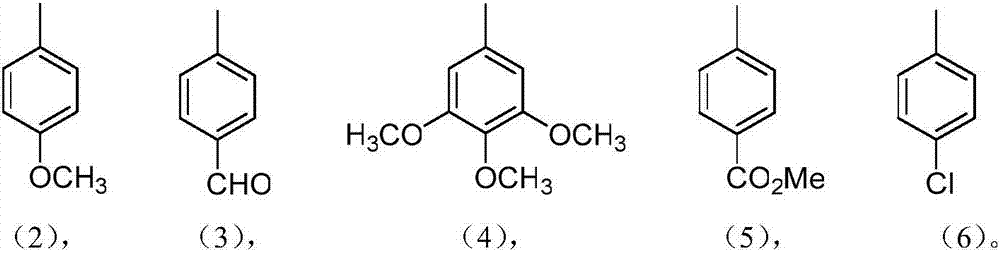
[0026] Weigh aminonorcarbrole (18mg, 0.03mmol) and 4-methoxybenzaldehyde (0.18mmol) in 35mL capped tube reactor, add new distilled toluene (3ml) in above-mentioned reactor, in the presence of oxygen Under the conditions of 110 ° C stirring reaction for 8 hours, follow the reaction with thin layer chromatography (TLC), after the reaction is complete, cool to room temperature, use 200-300 mesh silica gel for column chromatography, and use a volume ratio of 1:1 or 1 : 2 mixed solvents of dichloromethane and normal hexane as eluent, collect the solution of the 2nd elution point, the collected solution obtains dark brown product after concentrating and drying, promptly gets the product pyridine fused norcarba azole compound, the yield is 74%.
[0027] Spectrum characterization data are as follows: 1 H NMR (500MHz, CDCl 3 ,298K) δ: -0.69(d,J=4.0Hz,2H,pyrrH),1.07(d,J=4.0Hz,2H,pyrrH),1.21(d,J=4.0Hz,2H,pyrrH),1.76( s,6H,-CH 3 ),1.77(s,6H,-CH 3 ),1.79(d,J=4.0Hz,2H,pyrrH),1.92(d,J=5...
Embodiment 2
[0030] In this example, the same synthesis process as in Example 1 was adopted, and the raw material was changed to terephthalaldehyde in an amount of 0.18 mmol to obtain a dark brown product, that is, the product pyridine-fused norcarbrole compound with a yield of 73%.
[0031] Product spectral characterization data are as follows: 1 H NMR (500MHz, CDCl 3 ,298K)δ:-1.01(d,J=4.0Hz,2H,pyrrH),1.07(d,J=4.0Hz,2H,pyrrH),1.11(d,J=5.0Hz,2H,pyrrH),1.76( s,6H,-CH 3 ),1.78(br,6H(-CH 3 )+2H(pyrrH)), 1.92(d, J=5.0Hz, 2H, pyrrH), 2.11(d, J=5.0Hz, 2H, pyrrH), 2.16(s, 12H, -CH 3 ),2.59(s,12H,-CH 3 ), 5.74(d, J=9.0Hz, 2H, ArH), 5.85(s, 4H, ArH), 6.17(s, 4H, ArH), 6.76(d, J=9.0Hz, 2H, ArH), 9.09( s,1H,-CHO); 13 C NMR (125MHz, CDCl 3 ,298K)δ:17.60(-CH 3 ),17.64(-CH 3 ),20.5(-CH 3 ),20.9(-CH 3 ),114.2(pyrrC),114.3(pyrrC),117.9(pyrrC),120.0,121.8(pyrrC),122.5(pyrrC),125.6,125.7,127.4,127.5,127.7,127.9,132.3,133.4,134.6,134.7,135 (pyrrC), 136.8, 139.0, 148.0, 148.2, 148.4, 149.4, 153.8,...
Embodiment 3
[0034] This example uses basically the same synthesis process as Example 1, the raw material is changed to 3,4,5-trimethoxybenzaldehyde, the dosage is 0.18mmol, and the dark brown product is obtained, namely the product pyridine-fused norcarbrole compound , the yield was 80%.
[0035] Product spectral characterization data are as follows: 1 H NMR (500MHz, CDCl 3 ,298K) δ: -0.45(d,J=4.0Hz,2H,pyrrH),1.06(d,J=3.5Hz,2H,pyrrH),1.21(d,J=4.0Hz,2H,pyrrH),1.78( s,12H(-CH 3 )+2H(pyrrH)), 1.91(d, J=4.5Hz, 2H, pyrrH), 2.10(d, J=4.5Hz, 2H, pyrrH), 2.15(s, 12H, -CH 3 ),2.62(s,12H,-CH 3 ),2.84(s,3H,-OCH 3 ),3.58(s,6H,-OCH 3 ),4.65(s,2H,ArH),5.85(s,4H,ArH),6.20(s,4H,ArH); 13 C NMR (125MHz, CDCl 3 ,298K)δ:17.6(-CH 3 ),17.7(-CH 3 ),20.5(-CH 3 ),20.9(-CH 3 ),56.0(-OCH 3 ),60.1(-OCH 3 ),104.5,114.1(pyrrC),115.2(pyrrC),117.7(pyrrC),121.9(pyrrC),122.2(pyrrC),125.8,127.7,127.9,132.3,133.5,134.6,135.5(pyrrC),136.8,137.3, 139.1, 148.0, 148.1, 148.4, 151.1, 156.9, 167.7, 176.1; UV-vis (C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com