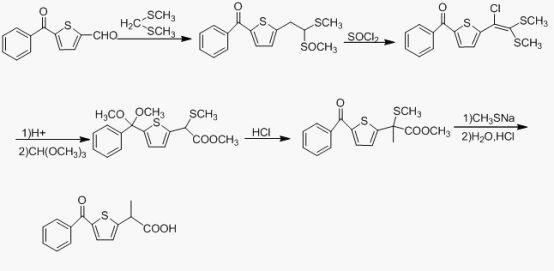
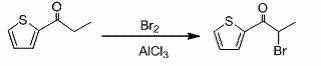Preparation method of tiaprofenic acid
A technology of tiaprofenic acid and thiophene, which is applied in the field of preparation of non-steroidal anti-inflammatory drug tiaprofenic acid, can solve the problems of difficult storage of tiaprofenic acid reaction raw materials, harsh reaction conditions, large environmental pollution, etc., and achieves convenient Industrial production, stable reaction conditions, and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
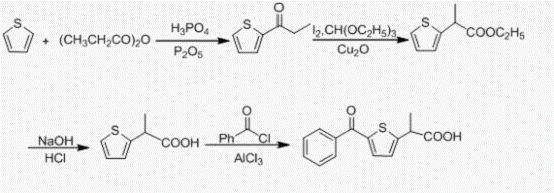
[0041] The synthesis of embodiment 1 2-propionyl thiophene
[0042]Add 32mL (0.4mol) of thiophene, 64mL (0.5mol) of propionic anhydride, 4mL of phosphoric acid and 0.8g (6mmol) of phosphorus pentoxide into a 250mL eggplant-shaped bottle at one time, and heat the oil bath to reflux at 95-100°C. After 20 minutes of reaction, the clarified system began to darken. With the prolongation of the reaction time, the color of the system gradually turned dark brown, and "white smoke" was continuously generated in the bottle. Stop the reaction after 6h-8h, cool the system to room temperature, add 100mL water and stir for 20min, extract with 3x50mL dichloromethane, combine the organic layer; stir the organic layer with 15% NaOH solution 100mL at room temperature for 1h until the upper aqueous solution of the system becomes alkaline, Then wash with 4x50mL saturated saline. Anhydrous sodium sulfate was dried overnight, filtered, and the solvent was distilled off under reduced pressure to ob...
Embodiment 2
[0043] Example 2 Synthesis of α-bromo-2-propionylthiophene
[0044] Add 14g (0.1mol) of 2-propionylthiophene and 60mL of refined ether solvent into a dry 250mL eggplant-shaped bottle. The temperature of the system was lowered to 0°C in an ice-salt bath, and 1.4 g (0.01 mol) of anhydrous aluminum trichloride catalyst was added. Add 8g (0.1mol) of bromine dropwise under stirring. When adding bromine dropwise, add 2 drops of bromine first, and then quickly add the remaining bromine dropwise after the color in the system fades away. At this time, the bromine added dropwise rapidly became colorless, and the generated hydrogen bromide was absorbed with 10% sodium hydroxide. After 10 minutes of dripping, the system is light red. The reaction was continued at this temperature for 30 min. The reaction was stopped, and the system was poured into a 200mL ice-water bath. At this time, the system changed from light red to light yellow, and stirred for several minutes. Wash with saturat...
Embodiment 3
[0046] Example 3 Synthesis of 2-(1-bromoethyl)-2-(2-methylthiophene)-1,3-dioxane
[0047] Add 44.0g (0.2mol) of α-bromo-2-propionylthiophene, 240mL of toluene and 4.4g of p-toluenesulfonic acid to a 500mL dry three-necked flask, heat up to 140°C in an oil bath, and vigorously reflux. After the temperature stabilized, start adding 2 equivalents of ethylene glycol. The temperature was refluxed for 12h, and the reaction was stopped. After the system was cooled to room temperature, it was washed with 3x40mL saturated brine. The upper layer was dried, filtered, and the solvent was evaporated to obtain 52.4 g of a light yellow oil, and the structure of the product was: , yield 99.3%, HPLC>99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com